How To Do Flame Test In Salt Analysis . a flame test is a complex phenomenon that is not fully explained. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. An easier way to perform the flame. in this video you will learn that , how to check positive ion if coloured flame. in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it).
from studylib.net
revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. An easier way to perform the flame. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. in this video you will learn that , how to check positive ion if coloured flame. a flame test is a complex phenomenon that is not fully explained. in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it).
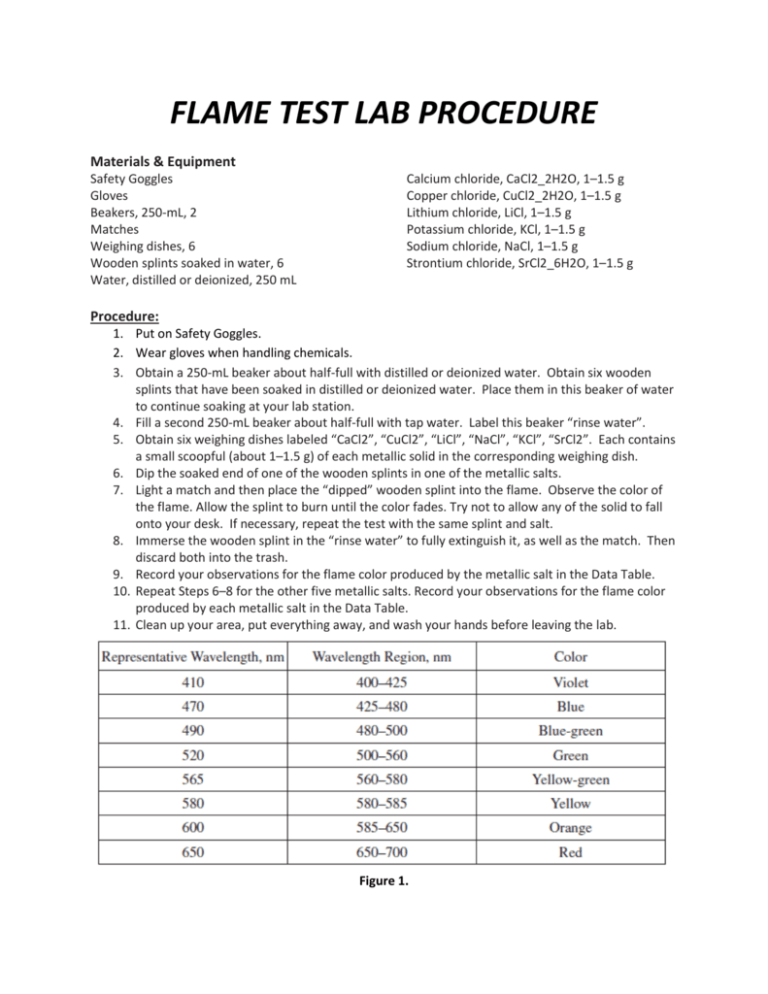
FLAME TEST LAB PROCEDURE
How To Do Flame Test In Salt Analysis in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. a flame test is a complex phenomenon that is not fully explained. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. An easier way to perform the flame. revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. in this video you will learn that , how to check positive ion if coloured flame. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it).
From www.youtube.com
salt analysis part 4 cobalt nitrate test charcoal cavity test How To Do Flame Test In Salt Analysis in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. a flame test is a complex phenomenon that is not fully explained. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). in. How To Do Flame Test In Salt Analysis.
From dxohmtoii.blob.core.windows.net
Flame Test Produce at James Miers blog How To Do Flame Test In Salt Analysis In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). it is widely used to detect and analyze the presence of certain elements in the given salt or compound. a. How To Do Flame Test In Salt Analysis.
From www.youtube.com
FLAME TEST ON SALT SAMPLES Na, Ba, K, Ca Cu and Sr test CHEMISTRY How To Do Flame Test In Salt Analysis An easier way to perform the flame. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). it is widely used to detect and analyze the presence of certain elements in the given salt or compound. in this classic science experiment, students report on the. How To Do Flame Test In Salt Analysis.
From www.pinterest.co.uk
Flame test colours The flame test is used to visually determine the How To Do Flame Test In Salt Analysis in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. An easier way to perform the flame. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. revision notes on flame tests for the edexcel igcse chemistry. How To Do Flame Test In Salt Analysis.
From pixels.com
Performing A Sodium Flame Test Photograph by Jerry Mason/science Photo How To Do Flame Test In Salt Analysis in this video you will learn that , how to check positive ion if coloured flame. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. the flame test is an analytical chemistry. How To Do Flame Test In Salt Analysis.
From ar.inspiredpencil.com
Flame Test Sodium How To Do Flame Test In Salt Analysis it is widely used to detect and analyze the presence of certain elements in the given salt or compound. a flame test is a complex phenomenon that is not fully explained. in this video you will learn that , how to check positive ion if coloured flame. In simple words, when a solution of metal salts, e.g.,. How To Do Flame Test In Salt Analysis.
From onlinecalculator.guru
Salt Analysis Formulas Complete List Formulae Sheet for Salt Analysis How To Do Flame Test In Salt Analysis revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. An easier way to perform the flame. a flame test is a complex phenomenon that is not fully. How To Do Flame Test In Salt Analysis.
From lessonlibciceronian.z22.web.core.windows.net
Science Experiments Involving Fire How To Do Flame Test In Salt Analysis in this video you will learn that , how to check positive ion if coloured flame. in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. . How To Do Flame Test In Salt Analysis.
From studylib.net
FLAME TEST LAB PROCEDURE How To Do Flame Test In Salt Analysis in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). the flame test is an analytical chemistry technique that helps identify elements in samples. How To Do Flame Test In Salt Analysis.
From www.science-revision.co.uk
Testing for cations How To Do Flame Test In Salt Analysis revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). the flame test is an analytical chemistry technique that helps identify elements in samples based. How To Do Flame Test In Salt Analysis.
From www.youtube.com
chemistry properties flametest Flame test for metal ions YouTube How To Do Flame Test In Salt Analysis if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). it is widely used to detect and analyze the presence of certain elements in the given salt or compound. An easier way to perform the flame. revision notes on flame tests for the edexcel igcse. How To Do Flame Test In Salt Analysis.
From sciencelessonsthatrock.com
Chemistry Flame Test Lab Science Lessons That Rock How To Do Flame Test In Salt Analysis a flame test is a complex phenomenon that is not fully explained. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). in this video you will learn that , how to check positive ion if coloured flame. it is widely used to detect. How To Do Flame Test In Salt Analysis.
From studylib.net
Flame Test Lab How To Do Flame Test In Salt Analysis a flame test is a complex phenomenon that is not fully explained. in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. An easier way to perform the flame. in this. How To Do Flame Test In Salt Analysis.
From studylib.net
Lab Flame Tests of Metal Salts How To Do Flame Test In Salt Analysis In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). An easier way to perform the flame. in this video you will learn that , how to check positive ion if. How To Do Flame Test In Salt Analysis.
From webapi.bu.edu
💐 Potassium chloride flame test. Difference Between Calcium Chloride How To Do Flame Test In Salt Analysis a flame test is a complex phenomenon that is not fully explained. in this video you will learn that , how to check positive ion if coloured flame. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. in this classic science experiment, students report on the. How To Do Flame Test In Salt Analysis.
From www.sciencecompany.com
Flame Test Chemical Kit With Five Chemicals from the Science Company. How To Do Flame Test In Salt Analysis a flame test is a complex phenomenon that is not fully explained. in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). revision. How To Do Flame Test In Salt Analysis.
From ditheodamme.com
Why Are Flame Tests Essential For Identifying Alkali Metals? How To Do Flame Test In Salt Analysis revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. An easier way to perform the flame. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. it is widely used to detect and analyze the presence of certain elements in the given. How To Do Flame Test In Salt Analysis.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind How To Do Flame Test In Salt Analysis a flame test is a complex phenomenon that is not fully explained. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. in this video you will learn that , how to check positive ion if coloured flame. An easier way to perform the flame. it is widely used to detect and. How To Do Flame Test In Salt Analysis.
From www.youtube.com
FLAME TEST group 5 salt analysis by Seema Makhijani YouTube How To Do Flame Test In Salt Analysis in this video you will learn that , how to check positive ion if coloured flame. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. a flame test is a complex phenomenon that is not fully explained. the flame test is an analytical chemistry technique that helps identify elements in samples. How To Do Flame Test In Salt Analysis.
From www.youtube.com
Qualitative Flame Test KCl Potassium Chloride YouTube How To Do Flame Test In Salt Analysis In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. in this video you will learn that , how to check positive ion if coloured flame. it is widely used to detect and. How To Do Flame Test In Salt Analysis.
From www.youtube.com
flame test salts 1 YouTube How To Do Flame Test In Salt Analysis in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it).. How To Do Flame Test In Salt Analysis.
From www.compoundchem.com
Metal Ion Flame Test Colours Chart Compound Interest How To Do Flame Test In Salt Analysis in this video you will learn that , how to check positive ion if coloured flame. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. in this. How To Do Flame Test In Salt Analysis.
From edu.rsc.org
Flame colours a demonstration Experiment RSC Education How To Do Flame Test In Salt Analysis if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). An easier way to perform the flame. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. In simple words, when a solution of metal salts, e.g.,. How To Do Flame Test In Salt Analysis.
From www.pinterest.co.uk
Effect of anion on flame colours of metal salts Chemistry classroom How To Do Flame Test In Salt Analysis if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. in this video you will learn that , how to check positive ion if coloured. How To Do Flame Test In Salt Analysis.
From hightechhigh-faithsdp.weebly.com
Flame Test Lab Faith's DP How To Do Flame Test In Salt Analysis a flame test is a complex phenomenon that is not fully explained. In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. it is widely used to detect and analyze the presence of certain elements in the given salt or compound. revision notes on flame tests for the edexcel igcse chemistry syllabus,. How To Do Flame Test In Salt Analysis.
From www.youtube.com
How to do flame test in salt analysis? YouTube How To Do Flame Test In Salt Analysis the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. An easier way to perform the flame. revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. In simple words, when a solution of metal salts, e.g., an aqueous. How To Do Flame Test In Salt Analysis.
From www.slideshare.net
CHEMISTRY Salt analysis class 12 How To Do Flame Test In Salt Analysis a flame test is a complex phenomenon that is not fully explained. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). revision notes on. How To Do Flame Test In Salt Analysis.
From homepage.ufp.pt
Pagina F (Termos) How To Do Flame Test In Salt Analysis if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). it is widely used to detect and analyze the presence of certain elements in the given salt or compound. revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts. How To Do Flame Test In Salt Analysis.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) How To Do Flame Test In Salt Analysis in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. An easier way to perform the flame. if the salt is colourless, perform a flame test. How To Do Flame Test In Salt Analysis.
From sciencenotes.org
Colored Fire Spray Bottles How To Do Flame Test In Salt Analysis in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). in this video you will learn that , how to check positive ion if. How To Do Flame Test In Salt Analysis.
From javapages.beanopolis.com
Flame Test Copper How To Do Flame Test In Salt Analysis in this video you will learn that , how to check positive ion if coloured flame. a flame test is a complex phenomenon that is not fully explained. An easier way to perform the flame. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. In simple words,. How To Do Flame Test In Salt Analysis.
From pixels.com
Flame Test Sequence Photograph by Science Photo Library Pixels How To Do Flame Test In Salt Analysis in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. in this video you will learn that , how to check positive ion if coloured flame. In. How To Do Flame Test In Salt Analysis.
From www.youtube.com
RC Unit 4 Demo Metal Salt Flame Test Using Methanol YouTube How To Do Flame Test In Salt Analysis a flame test is a complex phenomenon that is not fully explained. if the salt is colourless, perform a flame test first (since the presence of 3 different cations can be confirmed by it). In simple words, when a solution of metal salts, e.g., an aqueous solution of metal. in this classic science experiment, students report on. How To Do Flame Test In Salt Analysis.
From www.thoughtco.com
How to Do a Flame Test for Qualitative Analysis How To Do Flame Test In Salt Analysis in this video you will learn that , how to check positive ion if coloured flame. the flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. in this classic science experiment, students report on the colours produced when flame tests are carried out on different metal salts. . How To Do Flame Test In Salt Analysis.
From www.youtube.com
Flame Tests Chemistry Practicals YouTube How To Do Flame Test In Salt Analysis it is widely used to detect and analyze the presence of certain elements in the given salt or compound. a flame test is a complex phenomenon that is not fully explained. revision notes on flame tests for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. in this classic science experiment,. How To Do Flame Test In Salt Analysis.