Copper Sulfate Battery . A coordinating hydrogel is used as electrolyte. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. One of the simplest cells is the daniell cell. Copper sulfate and zinc sulfate. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. Great for science fairs and similar projects this battery can be used to. A zinc sulfate solution is floated on top of the copper sulfate solution; Then a zinc electrode is placed in the zinc sulfate solution.
from nationaldefensepac.org
A zinc sulfate solution is floated on top of the copper sulfate solution; We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Then a zinc electrode is placed in the zinc sulfate solution. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. Copper sulfate and zinc sulfate. A coordinating hydrogel is used as electrolyte. Great for science fairs and similar projects this battery can be used to. One of the simplest cells is the daniell cell. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution.
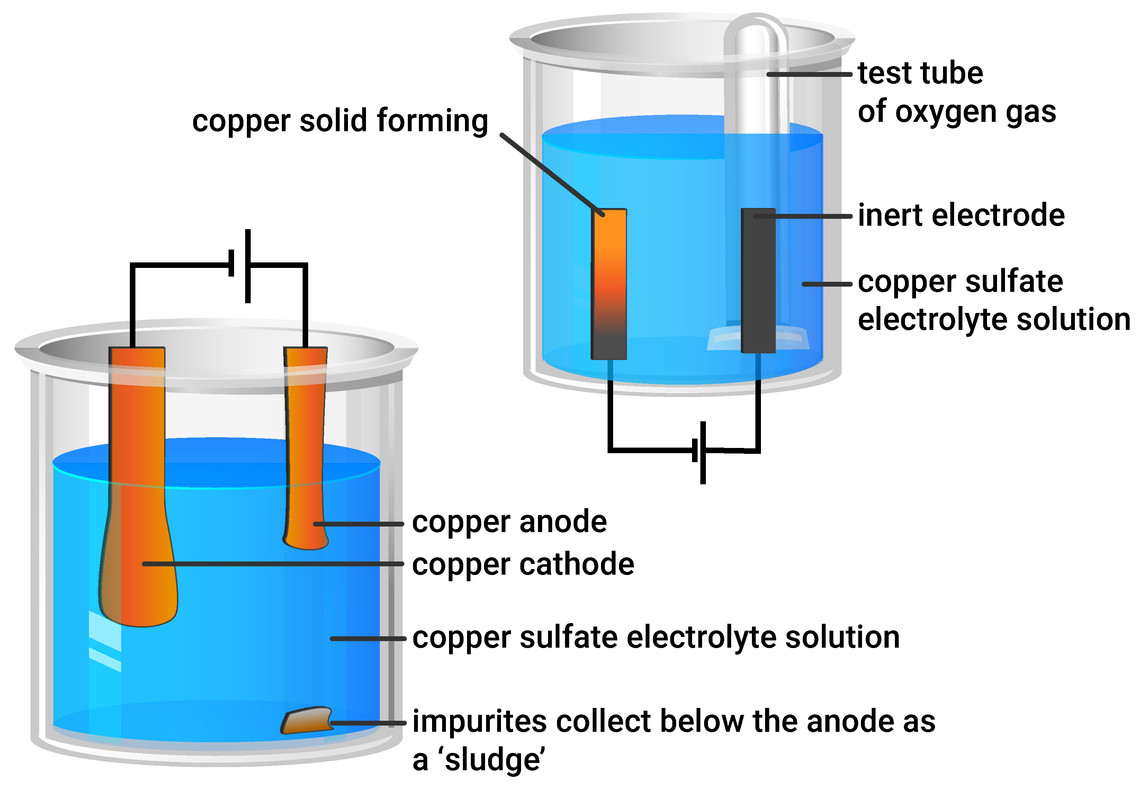
Principle Of Electrolysis Of Copper Sulfate Electrolyte, 57 OFF
Copper Sulfate Battery One of the simplest cells is the daniell cell. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Great for science fairs and similar projects this battery can be used to. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. One of the simplest cells is the daniell cell. A coordinating hydrogel is used as electrolyte. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. Copper sulfate and zinc sulfate. Then a zinc electrode is placed in the zinc sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such.
From www.thoughtco.com
What Is Electroplating and How Does It Work? Copper Sulfate Battery One of the simplest cells is the daniell cell. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such. Copper sulfate and zinc sulfate. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Great for science fairs and similar projects this battery can. Copper Sulfate Battery.
From www.youtube.com
single displacement reaction of Copper from Copper Sulphate Solution by Copper Sulfate Battery A coordinating hydrogel is used as electrolyte. Copper sulfate and zinc sulfate. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. One of the simplest cells is the daniell cell. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and. Copper Sulfate Battery.
From colouremployer8.gitlab.io
Marvelous Copper Zinc Battery Reaction Cie Chemistry A Level Syllabus Copper Sulfate Battery A coordinating hydrogel is used as electrolyte. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. Then a zinc electrode is placed in the zinc sulfate solution. This approach, currently investigated by. Copper Sulfate Battery.
From chem2u.blogspot.com
chem2U Electrolysis of Copper(II) Sulphate Copper Sulfate Battery We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Then a zinc electrode is placed in the zinc sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; Copper sulfate and zinc sulfate. One of the simplest cells is the daniell cell. A coordinating hydrogel is used. Copper Sulfate Battery.
From nationaldefensepac.org
Principle Of Electrolysis Of Copper Sulfate Electrolyte, 57 OFF Copper Sulfate Battery A coordinating hydrogel is used as electrolyte. A zinc sulfate solution is floated on top of the copper sulfate solution; Copper sulfate and zinc sulfate. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design. Copper Sulfate Battery.
From www.livmats.uni-freiburg.de
livMatS Aluminiumion batteries with improved storage capacity Copper Sulfate Battery Copper sulfate and zinc sulfate. One of the simplest cells is the daniell cell. Great for science fairs and similar projects this battery can be used to. Then a zinc electrode is placed in the zinc sulfate solution. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. A zinc. Copper Sulfate Battery.
From www.verywellhealth.com
Copper Sulfate Uses, Benefits, and Warnings Copper Sulfate Battery Copper sulfate and zinc sulfate. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such. A coordinating hydrogel is used as electrolyte. Then a zinc electrode is placed in the zinc sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; Great for science fairs and. Copper Sulfate Battery.
From www.youtube.com
DEMONSTRATING USING COPPER(II) SULFATE TO DETECT WATER A REVERSIBLE Copper Sulfate Battery Then a zinc electrode is placed in the zinc sulfate solution. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. This approach, currently investigated by our group, aims to demonstrate the improved. Copper Sulfate Battery.
From www.shimico.com
Copper Sulfate and the methods of production Shimico blog Copper Sulfate Battery We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Then a zinc electrode is placed in the zinc sulfate solution. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such. Copper sulfides (cu x s) are widely used as the promising electrode materials. Copper Sulfate Battery.
From socratic.org
What happens on the cathode during the electrolysis of a copper(II Copper Sulfate Battery Then a zinc electrode is placed in the zinc sulfate solution. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Copper sulfate and zinc sulfate. Great for science fairs and similar projects this battery can be used to. Copper sulfides (cu x s) are widely used as the promising electrode materials for. Copper Sulfate Battery.
From homebatterybank.com
Is Corrosion on my Car Battery Normal? Home Battery Bank Copper Sulfate Battery We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. One of the simplest cells is the daniell cell. Great for science fairs and similar projects this battery can be used to. A. Copper Sulfate Battery.
From byjus.com
Explain the electrolysis of copper sulphate solution with the help of a Copper Sulfate Battery It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. Then a zinc electrode is placed in the zinc sulfate solution. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. We make. Copper Sulfate Battery.
From sciencenotes.org
How to Make Copper Sulfate (Copper Sulphate) Copper Sulfate Battery We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. A zinc sulfate solution is floated on top of the copper sulfate solution; Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. Great for science fairs and similar projects this battery can. Copper Sulfate Battery.
From www.youtube.com
Copper Sulfate Crystals Forming under the Microscope YouTube Copper Sulfate Battery Great for science fairs and similar projects this battery can be used to. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. A zinc sulfate solution is floated on top of the copper sulfate solution; This approach, currently investigated by our group,. Copper Sulfate Battery.
From sci-toys.com
Chapter 3 Electrochemistry Make homemade batteries in your kitchen Copper Sulfate Battery A coordinating hydrogel is used as electrolyte. One of the simplest cells is the daniell cell. Copper sulfate and zinc sulfate. Great for science fairs and similar projects this battery can be used to. Then a zinc electrode is placed in the zinc sulfate solution. It is possible to construct this battery by placing a copper electrode at the bottom. Copper Sulfate Battery.
From www.dreamstime.com
Electrolysis of Copper Sulfate Solution with Impure Copper Anode and Copper Sulfate Battery Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. Then a zinc electrode is placed in the zinc sulfate solution. Great for science fairs and similar projects this battery can be used to. A zinc sulfate solution is floated on top of the copper sulfate solution; It is possible. Copper Sulfate Battery.
From pixels.com
Zinccopper Battery Photograph by Andrew Lambert Photography Copper Sulfate Battery We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. Copper sulfate and zinc sulfate. This approach, currently investigated by our group, aims to demonstrate the. Copper Sulfate Battery.
From fixcarbattery.blogspot.com
Recondition Car battery Magnesium copper water battery Copper Sulfate Battery Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. Then a zinc electrode is placed in the zinc sulfate solution. Great for science fairs and similar projects this battery can be used to. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of. Copper Sulfate Battery.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Copper Sulfate Battery Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. One of the simplest cells is the daniell cell. This approach, currently investigated. Copper Sulfate Battery.
From www.dreamstime.com
Electroplating with Copper Using Copper Sulfate Electrolyte Stock Copper Sulfate Battery Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. Then a zinc electrode is placed in the zinc sulfate solution. A coordinating hydrogel is used as electrolyte. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such. Great for science. Copper Sulfate Battery.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID3293570 Copper Sulfate Battery Then a zinc electrode is placed in the zinc sulfate solution. A coordinating hydrogel is used as electrolyte. Great for science fairs and similar projects this battery can be used to. Copper sulfate and zinc sulfate. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. One of the simplest. Copper Sulfate Battery.
From helpiks.org
ELECTROLYSIS OF AQUEOUSSOLUTIONS Copper Sulfate Battery Copper sulfate and zinc sulfate. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. Copper sulfides (cu x s) are widely used as. Copper Sulfate Battery.
From www.basinc.com
BASi® Saturated Copper Sulfate Storage/Refill Solution Copper Sulfate Battery Then a zinc electrode is placed in the zinc sulfate solution. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. One of the simplest cells is the daniell cell. A zinc sulfate solution is floated on top of the copper sulfate solution; A coordinating hydrogel is used as electrolyte. Great for science. Copper Sulfate Battery.
From www.researchgate.net
Schematic illustration and operating mechanism of rechargeable ZnCu Copper Sulfate Battery Then a zinc electrode is placed in the zinc sulfate solution. Copper sulfate and zinc sulfate. One of the simplest cells is the daniell cell. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium. Copper Sulfate Battery.
From www.youtube.com
Make a Copper sulfate and zinc battery "Gravity" design YouTube Copper Sulfate Battery It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. A coordinating hydrogel is used as electrolyte. Great for science fairs and similar projects this battery can be used to. This approach, currently investigated by our group, aims to demonstrate the improved reactivity. Copper Sulfate Battery.
From www.youtube.com
Electrolysis of Copper Sulphate YouTube Copper Sulfate Battery We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Then a zinc electrode is placed in the zinc sulfate solution. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. A zinc sulfate solution is. Copper Sulfate Battery.
From answertion.com
What is battery corrosion material? Q&A Answertion Copper Sulfate Battery A coordinating hydrogel is used as electrolyte. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. Great for science fairs and similar projects this battery can be used to. One of the simplest cells is the daniell cell. Copper sulfate and zinc sulfate. This approach, currently investigated by our. Copper Sulfate Battery.
From www.youtube.com
Electrolysis Of Copper(ii) Sulphate Using Copper Electrodes YouTube Copper Sulfate Battery A coordinating hydrogel is used as electrolyte. A zinc sulfate solution is floated on top of the copper sulfate solution; Copper sulfate and zinc sulfate. Then a zinc electrode is placed in the zinc sulfate solution. One of the simplest cells is the daniell cell. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary. Copper Sulfate Battery.
From www.youtube.com
Reaction of Copper Sulphate (CuSO4) with Sodium Hydroxide (NaOH) YouTube Copper Sulfate Battery A coordinating hydrogel is used as electrolyte. A zinc sulfate solution is floated on top of the copper sulfate solution; We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Great for science fairs and similar projects this battery can be used to. This approach, currently investigated by our group, aims to demonstrate. Copper Sulfate Battery.
From www.youtube.com
COPPER SULPHATE USES BENEFITS (कॉपर सल्फेट) YouTube Copper Sulfate Battery Copper sulfate and zinc sulfate. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. Then a zinc electrode is placed in the zinc sulfate solution.. Copper Sulfate Battery.
From electro-lysis.blogspot.com
Electro electricity lysisbreak down Selective Discharge and the Copper Sulfate Battery This approach, currently investigated by our group, aims to demonstrate the improved reactivity versus lithium of a compound such. We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Copper sulfate and zinc sulfate. One of the simplest cells is the daniell cell. It is possible to construct this battery by placing a. Copper Sulfate Battery.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Copper Sulfate Battery Then a zinc electrode is placed in the zinc sulfate solution. A coordinating hydrogel is used as electrolyte. Great for science fairs and similar projects this battery can be used to. One of the simplest cells is the daniell cell. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich.. Copper Sulfate Battery.
From www.vecteezy.com
Electrolysis of copper sulfate solution with impure copper anode and Copper Sulfate Battery A zinc sulfate solution is floated on top of the copper sulfate solution; We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. Great for science fairs and similar projects this battery can be used to. Copper sulfate and zinc sulfate. A coordinating hydrogel is used as electrolyte. Then a zinc electrode is. Copper Sulfate Battery.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Copper Sulfate Battery We make the classic copper sulfate and zinc battery using the incredibly easy gravity battery design approach. A zinc sulfate solution is floated on top of the copper sulfate solution; A coordinating hydrogel is used as electrolyte. Copper sulfides (cu x s) are widely used as the promising electrode materials for secondary batteries because of the rich. Great for science. Copper Sulfate Battery.
From education.seattlepi.com
What Happens in a Battery if Zinc Sulfate Is Mixed With Copper Sulfate Copper Sulfate Battery Great for science fairs and similar projects this battery can be used to. A zinc sulfate solution is floated on top of the copper sulfate solution; It is possible to construct this battery by placing a copper electrode at the bottom of a jar and covering the metal with a copper sulfate solution. We make the classic copper sulfate and. Copper Sulfate Battery.