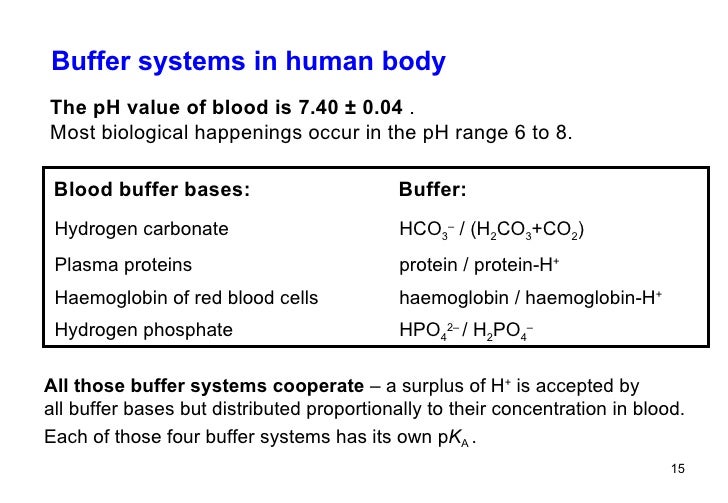
Define Buffer And Discuss The Role Of Buffers In The Body . The buffer systems in the human body are extremely efficient, and different systems work at different rates. Buffers are substances that help maintain the ph of a solution within a specific range. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. A buffer is a solution which. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. As an example, blood has a ph of 7.4. Buffer systems in the body. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. There are two ways to achieve a ph of 7.4. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. Identify the six ions most important to the function of the body. The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph.
from www.slideshare.net
The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. Identify the six ions most important to the function of the body. A buffer is a solution which. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. There are two ways to achieve a ph of 7.4. Buffer systems in the body.
02 hydrolysis. buffers__colloids
Define Buffer And Discuss The Role Of Buffers In The Body A buffer is a solution which. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffers are substances that help maintain the ph of a solution within a specific range. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. Buffer systems in the body. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. As an example, blood has a ph of 7.4. The buffer systems in the human body are extremely efficient, and different systems work at different rates. A buffer is a solution which. There are two ways to achieve a ph of 7.4. Identify the six ions most important to the function of the body. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph.
From studyloadstones.z21.web.core.windows.net
Why Are Buffers Important To Homeostasis Define Buffer And Discuss The Role Of Buffers In The Body The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. As an example, blood has a ph of 7.4. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. Identify the six ions most important to the function of the body. Define. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideshare.net
Buffer system Define Buffer And Discuss The Role Of Buffers In The Body Define plasma osmolality and identify two ways in which plasma osmolality is maintained. A buffer is a solution which. Identify the six ions most important to the function of the body. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. The role of a buffer in the body can be determined by comparing two aqueous. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Define Buffer And Discuss The Role Of Buffers In The Body The buffer systems in the human body are extremely efficient, and different systems work at different rates. Buffer systems in the body. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. There are two ways to achieve a ph of 7.4. Buffers are substances that help maintain the ph of a. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Define Buffer And Discuss The Role Of Buffers In The Body A buffer is a solution which. As an example, blood has a ph of 7.4. Buffers are substances that help maintain the ph of a solution within a specific range. Buffer systems in the body. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. Define plasma osmolality and identify two ways. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Define Buffer And Discuss The Role Of Buffers In The Body A buffer is a solution which. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. As an. Define Buffer And Discuss The Role Of Buffers In The Body.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Define Buffer And Discuss The Role Of Buffers In The Body Buffers are substances that help maintain the ph of a solution within a specific range. Identify the six ions most important to the function of the body. There are two ways to achieve a ph of 7.4. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. They work by absorbing or releasing hydrogen. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.studocu.com
Chemistry of buffers and buffers in our body Chemistry of buffers and Define Buffer And Discuss The Role Of Buffers In The Body A buffer is a solution which. Buffers are substances that help maintain the ph of a solution within a specific range. The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. As an example, blood has a. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.youtube.com
Acid Base Physiology Part One Basics Buffers Renal Physiology Define Buffer And Discuss The Role Of Buffers In The Body There are two ways to achieve a ph of 7.4. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. A buffer is a solution which. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. The. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Define Buffer And Discuss The Role Of Buffers In The Body There are two ways to achieve a ph of 7.4. Identify the six ions most important to the function of the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Buffers are substances that help maintain the ph of a solution within a specific range. The role of a buffer in. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.tffn.net
What is a Buffer in Science? Exploring the Role of Buffers in Chemistry Define Buffer And Discuss The Role Of Buffers In The Body A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. There are two ways to achieve a ph of 7.4. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. A buffer is a solution which. Buffer systems in the body. As an example, blood has. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.youtube.com
Role of buffers in biological system YouTube Define Buffer And Discuss The Role Of Buffers In The Body Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. The buffer systems in the. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Define Buffer And Discuss The Role Of Buffers In The Body As an example, blood has a ph of 7.4. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. A buffer is a solution which. Buffers are substances that help maintain the ph of a solution within. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.osmosis.org
Physiologic pH and buffers Video, Anatomy & Definition Osmosis Define Buffer And Discuss The Role Of Buffers In The Body A buffer is a solution which. Buffers are substances that help maintain the ph of a solution within a specific range. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. Describe the chemistry of buffer. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideshare.net
Buffers in the body Define Buffer And Discuss The Role Of Buffers In The Body They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. Buffer systems in the body. Buffers are substances that help maintain the ph of a solution within a specific range. A buffer is a solution which. It takes only seconds for the chemical. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Define Buffer And Discuss The Role Of Buffers In The Body Buffer systems in the body. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. As an example, blood has a ph of 7.4. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. The role of a buffer in the body can be determined by. Define Buffer And Discuss The Role Of Buffers In The Body.
From poorvi77.blogspot.com
Poorveez ParadyzMedimuseion major buffer systems in the body Define Buffer And Discuss The Role Of Buffers In The Body It takes only seconds for the chemical buffers in the blood to make adjustments to ph. There are two ways to achieve a ph of 7.4. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. Buffers are substances that help maintain the ph of a solution within a specific range. A buffer is a solution. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Define Buffer And Discuss The Role Of Buffers In The Body They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. As an example, blood has a ph of 7.4. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. A buffer is a solution. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideshare.net
02 hydrolysis. buffers__colloids Define Buffer And Discuss The Role Of Buffers In The Body The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. Define plasma osmolality and identify two ways in which plasma osmolality is maintained. As an example, blood has a ph of 7.4. A buffer is a solution containing substances which have the ability to minimise changes in ph when an. Define Buffer And Discuss The Role Of Buffers In The Body.
From slidetodoc.com
Role of Buffers Role of Buffers Introduction Journals Define Buffer And Discuss The Role Of Buffers In The Body A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. As an example, blood has a ph of 7.4. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. Buffer systems in the body. Identify the six ions most important to the function of the. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Homeostasis PowerPoint Presentation, free download ID1138529 Define Buffer And Discuss The Role Of Buffers In The Body Identify the six ions most important to the function of the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. The role of a buffer in the body can be determined by comparing two aqueous solutions with. Define Buffer And Discuss The Role Of Buffers In The Body.
From slideplayer.com
Define pH and Buffers List the sources of hydrogen ions in the body Define Buffer And Discuss The Role Of Buffers In The Body Identify the six ions most important to the function of the body. There are two ways to achieve a ph of 7.4. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. A buffer is a solution which. Buffer systems in the body. They work by absorbing or releasing. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideshare.net
Buffer system Define Buffer And Discuss The Role Of Buffers In The Body Identify the six ions most important to the function of the body. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The respiratory tract can adjust the blood ph upward in. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.youtube.com
Physiological Buffers YouTube Define Buffer And Discuss The Role Of Buffers In The Body Buffers are substances that help maintain the ph of a solution within a specific range. Identify the six ions most important to the function of the body. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. A buffer is a solution which. It takes only seconds for the chemical buffers in the blood to make. Define Buffer And Discuss The Role Of Buffers In The Body.
From slidetodoc.com
Renal Physiology 10 AcidBase Balance 2 Buffers System Define Buffer And Discuss The Role Of Buffers In The Body Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. Buffer systems in the body. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. The buffer systems in the human body are extremely efficient, and different systems work at different rates. A buffer is a solution which. There are two ways. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Define Buffer And Discuss The Role Of Buffers In The Body Buffers are substances that help maintain the ph of a solution within a specific range. The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. Identify the six ions most important to the function of the. Define Buffer And Discuss The Role Of Buffers In The Body.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Define Buffer And Discuss The Role Of Buffers In The Body A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. There are two ways to achieve a ph of 7.4. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. Buffers are substances that help maintain the ph of a solution. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Define Buffer And Discuss The Role Of Buffers In The Body The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Describe the chemistry of buffer mechanisms and explain their. Define Buffer And Discuss The Role Of Buffers In The Body.
From present5.com
Fundamentals of General Organic and Biological Chemistry 6 Define Buffer And Discuss The Role Of Buffers In The Body It takes only seconds for the chemical buffers in the blood to make adjustments to ph. As an example, blood has a ph of 7.4. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base.. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 Define Buffer And Discuss The Role Of Buffers In The Body The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffer systems in the body. As an example, blood has a ph. Define Buffer And Discuss The Role Of Buffers In The Body.
From dokumen.tips
(PPT) Blood Buffers Module H Malley pages 120126. Objectives Define a Define Buffer And Discuss The Role Of Buffers In The Body Identify the six ions most important to the function of the body. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. Buffer systems in the body. Describe the chemistry of buffer mechanisms. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 Define Buffer And Discuss The Role Of Buffers In The Body It takes only seconds for the chemical buffers in the blood to make adjustments to ph. Describe the chemistry of buffer mechanisms and explain their relevant roles in the body. Buffers are substances that help maintain the ph of a solution within a specific range. There are two ways to achieve a ph of 7.4. The respiratory tract can adjust. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Define Buffer And Discuss The Role Of Buffers In The Body The buffer systems in the human body are extremely efficient, and different systems work at different rates. The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. There are two ways to achieve a ph of. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 Define Buffer And Discuss The Role Of Buffers In The Body Identify the six ions most important to the function of the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. As an example, blood has a ph of 7.4. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. The role of a buffer in the body. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Define Buffer And Discuss The Role Of Buffers In The Body The buffer systems in the human body are extremely efficient, and different systems work at different rates. The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. A buffer is a solution which. It takes only seconds for the chemical buffers in the blood to make adjustments to ph. As. Define Buffer And Discuss The Role Of Buffers In The Body.
From www.pinterest.com
chemical and physiological pH buffers Biochemistry notes, Nursing Define Buffer And Discuss The Role Of Buffers In The Body The respiratory tract can adjust the blood ph upward in minutes by exhaling co 2 from the body. A buffer is a solution which. The role of a buffer in the body can be determined by comparing two aqueous solutions with the same ph. It takes only seconds for the chemical buffers in the blood to make adjustments to ph.. Define Buffer And Discuss The Role Of Buffers In The Body.