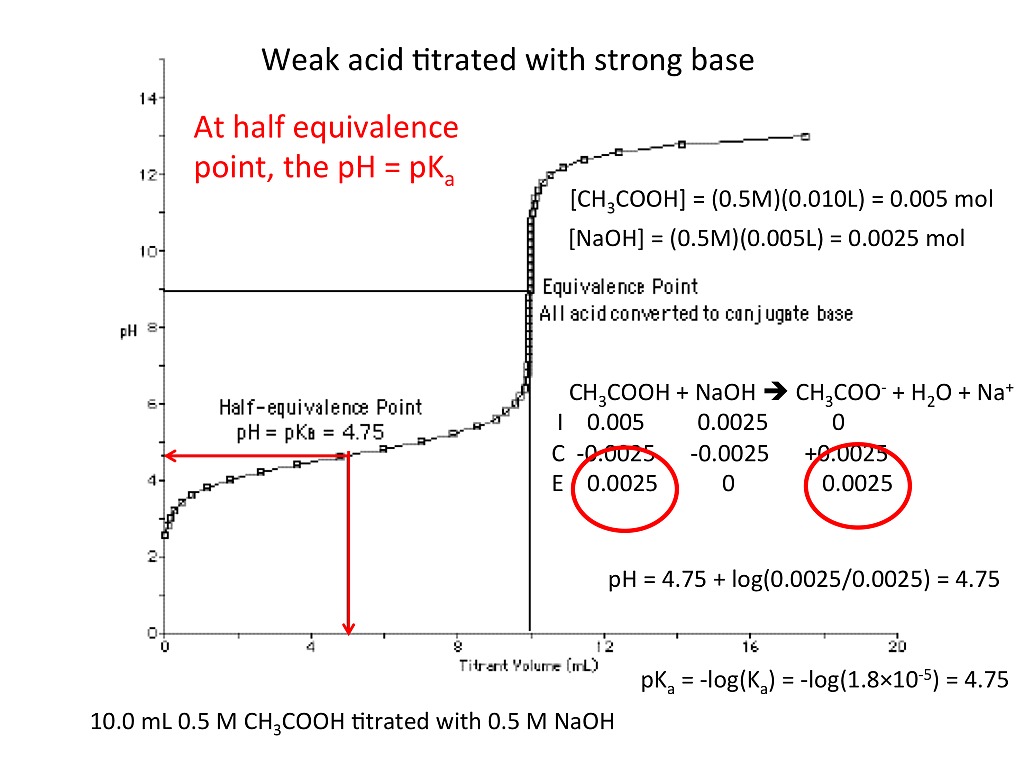
Titration Curve And Concentrations . The titration curve is a graphical representation of the ph or other property changes during a titration experiment. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The end point of a titration is the point at which an indicator changes. It provides valuable information about the reaction under study and helps understand the results obtained. A titration curve is a graphical representation of the ph of a solution during a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.
from www.showme.com
It is based on the neutralization reaction, where an acid and a base react to form water and a salt. It provides valuable information about the reaction under study and helps understand the results obtained. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or.
Titration Curve Explained Science, Chemistry ShowMe
Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The end point of a titration is the point at which an indicator changes. It provides valuable information about the reaction under study and helps understand the results obtained. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid.
From www.chegg.com
Solved The following figure shows the titration curve Titration Curve And Concentrations Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. The titration curve is. Titration Curve And Concentrations.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve And Concentrations It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid. Titration Curve And Concentrations.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve And Concentrations Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A titration curve is a graphical representation of the ph of a solution during a titration. The end point. Titration Curve And Concentrations.
From moodle.tau.ac.il
AcidBase Titration Curves Titration Curve And Concentrations It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It provides valuable information about the reaction under study and helps understand the results obtained. The end. Titration Curve And Concentrations.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve And Concentrations As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. Titration curves show how the ph of an acidic or basic solution changes as a basic or. Titration Curve And Concentrations.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Curve And Concentrations Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. As seen in the chapter on the stoichiometry of chemical reactions,. Titration Curve And Concentrations.
From saylordotorg.github.io
AcidBase Titrations Titration Curve And Concentrations A titration curve is a graphical representation of the ph of a solution during a titration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base. Titration Curve And Concentrations.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. As seen in the chapter on the stoichiometry. Titration Curve And Concentrations.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Curve And Concentrations It is based on the neutralization reaction, where an acid and a base react to form water and a salt. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It. Titration Curve And Concentrations.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve And Concentrations Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of. Titration Curve And Concentrations.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Curve And Concentrations Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps understand the results obtained. As seen in. Titration Curve And Concentrations.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve And Concentrations Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Titration curves show how the ph of an acidic or basic. Titration Curve And Concentrations.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher ionic equilibrium titration curves Titration Curve And Concentrations Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. The end point of a titration is the point at which an indicator changes.. Titration Curve And Concentrations.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve And Concentrations Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration curve is a graphical representation of the ph of a solution during. Titration Curve And Concentrations.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve And Concentrations The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It provides valuable information about the reaction under study and helps understand the results obtained. As seen in the. Titration Curve And Concentrations.
From srkwxfutjcwku.blogspot.com
How To Find Initial Concentration From Titration Curve The initial Titration Curve And Concentrations Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It provides valuable information about the reaction under study and helps understand the results obtained. A titration curve is a graphical representation of the ph of a solution during a titration. As seen in the chapter on. Titration Curve And Concentrations.
From sansona.github.io
Titrations Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. Titration Curve And Concentrations.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. It provides valuable information about the reaction under study and helps understand the results obtained. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a. Titration Curve And Concentrations.
From www.researchgate.net
21 15 N chemical shift titration plots of histidine side chains in the Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. A titration curve is a graphical representation of the ph of a solution during a titration. As seen in the chapter on the stoichiometry of chemical. Titration Curve And Concentrations.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Curve And Concentrations Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. It provides valuable information about the reaction under study and helps understand the results obtained. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution. Titration Curve And Concentrations.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Sketch out a plot representing the titration. Titration Curve And Concentrations.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for. Titration Curve And Concentrations.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve And Concentrations It provides valuable information about the reaction under study and helps understand the results obtained. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The end point of a titration is the point at which an indicator changes. The titration curve is a. Titration Curve And Concentrations.
From edurev.in
Graphical Representation of Titration Curves Chemistry for JAMB PDF Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. It provides valuable information about the reaction under study and helps understand the results obtained. Titration curves show how the ph of an. Titration Curve And Concentrations.
From www.animalia-life.club
Titration Curve Amino Acid Titration Curve And Concentrations A titration curve is a graphical representation of the ph of a solution during a titration. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. It is based on the neutralization reaction, where an acid and a base react to form water and. Titration Curve And Concentrations.
From www.researchgate.net
Alkalinity Titration Curve Download Scientific Diagram Titration Curve And Concentrations As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The titration curve is a graphical representation of the ph or other property changes. Titration Curve And Concentrations.
From www.researchgate.net
Conductometric titration curve of trospium chloride (12.5mg) vs Volume Titration Curve And Concentrations The titration curve is a graphical representation of the ph or other property changes during a titration experiment. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. The end point of a titration is the point at which an indicator changes. A titration curve is a. Titration Curve And Concentrations.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve And Concentrations It provides valuable information about the reaction under study and helps understand the results obtained. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which. Titration Curve And Concentrations.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. A titration curve is a graphical representation of the ph of a solution during a titration. Sketch out a plot representing the titration of a weak. Titration Curve And Concentrations.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve And Concentrations Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. It is based on the neutralization reaction, where an acid and. Titration Curve And Concentrations.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve And Concentrations Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information. Titration Curve And Concentrations.
From www.vrogue.co
The Graphs Labeled A And B Show The Titration Curves vrogue.co Titration Curve And Concentrations As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by a strong acid. A titration curve is a graphical representation of the ph of. Titration Curve And Concentrations.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Curve And Concentrations Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. It provides valuable information about the reaction under study and helps understand the results obtained. As seen. Titration Curve And Concentrations.
From www.youtube.com
Acid Base Titration Curves Simplified YouTube Titration Curve And Concentrations The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A titration curve is a graphical representation of the ph of a solution during a titration. It is based on the neutralization reaction, where an acid and a base react to form water and a salt. As seen in the chapter on. Titration Curve And Concentrations.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve And Concentrations The end point of a titration is the point at which an indicator changes. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. Titration Curve And Concentrations.