Electron Configuration School School Public School . The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Electrons and the periodic table part 3a: Electron configurations describe where electrons are located around the nucleus of an atom. Electron configuration of hydrogen (h) 1s 1:. Electron configuration 1s 2 2s 2 2p 2. For example, the electron configuration of lithium,. The bohr model of an atom. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. The shorthand electron configuration for fe is [ar]4s 2 3d 6. Use the periodic table to determine the number of valence electrons in an atom of a certain element. Write the element symbol and place the dots (valence electrons) on. Shorthand electron configuration full electron configuration electron shell arrangement; Following the 2s sublevel is the 2p, and p sublevels always. Electrons and the periodic table part.
from www.tes.com
Electrons and the periodic table part 3a: Shorthand electron configuration full electron configuration electron shell arrangement; The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Electrons and the periodic table part. Electron configurations describe where electrons are located around the nucleus of an atom. Electron configuration of hydrogen (h) 1s 1:. Following the 2s sublevel is the 2p, and p sublevels always. For example, the electron configuration of lithium,. Use the periodic table to determine the number of valence electrons in an atom of a certain element.
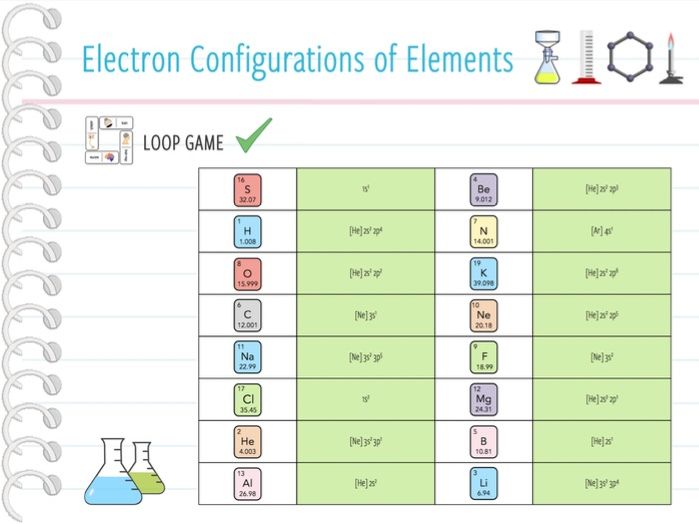
Electron Configuration of Elements Loop Game (KS4) by anjacschmidt
Electron Configuration School School Public School For example, the electron configuration of lithium,. Use the periodic table to determine the number of valence electrons in an atom of a certain element. The bohr model of an atom. Electron configuration of hydrogen (h) 1s 1:. Electron configuration 1s 2 2s 2 2p 2. Electrons and the periodic table part 3a: Electron configurations describe where electrons are located around the nucleus of an atom. Write the element symbol and place the dots (valence electrons) on. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Shorthand electron configuration full electron configuration electron shell arrangement; The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. Following the 2s sublevel is the 2p, and p sublevels always. For example, the electron configuration of lithium,. Electrons and the periodic table part. The shorthand electron configuration for fe is [ar]4s 2 3d 6.
From www.youtube.com
Drawing electron configuration diagrams Chemistry for All The Fuse Electron Configuration School School Public School Electron configuration of hydrogen (h) 1s 1:. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. Electron configuration 1s 2 2s 2 2p 2. Shorthand electron configuration full electron configuration electron shell arrangement; The shorthand electron configuration for fe is [ar]4s 2 3d. Electron Configuration School School Public School.
From worksheetstinifloclaniv9.z21.web.core.windows.net
Chemistry Electron Configuration Worksheets Electron Configuration School School Public School Electron configuration of hydrogen (h) 1s 1:. Electron configuration 1s 2 2s 2 2p 2. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. Electrons and the periodic table part 3a: The bohr model of an atom. Electrons and the periodic table part.. Electron Configuration School School Public School.
From www.tes.com
Electron Configuration of Elements Loop Game (KS4) by anjacschmidt Electron Configuration School School Public School Electrons and the periodic table part 3a: Electron configuration of hydrogen (h) 1s 1:. Write the element symbol and place the dots (valence electrons) on. Electrons and the periodic table part. Shorthand electron configuration full electron configuration electron shell arrangement; The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons. Electron Configuration School School Public School.
From www.pinterest.com
Electron Configuration Chart for the Elements Electron configuration Electron Configuration School School Public School The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. Following the 2s sublevel is the 2p, and p sublevels always. Write the element symbol and place the dots (valence electrons) on. For example, the electron configuration of lithium,. Electron configuration 1s 2 2s. Electron Configuration School School Public School.
From www.pinterest.co.uk
High School Chemistry Core Concept Cheat Sheet 13 Electron Electron Configuration School School Public School Write the element symbol and place the dots (valence electrons) on. Shorthand electron configuration full electron configuration electron shell arrangement; Electron configuration 1s 2 2s 2 2p 2. Following the 2s sublevel is the 2p, and p sublevels always. Electron configuration of hydrogen (h) 1s 1:. For example, the electron configuration of lithium,. Electrons and the periodic table part 3a:. Electron Configuration School School Public School.
From teachsimple.com
The Best Electron Configuration Practice Worksheet The Teach Simple Blog Electron Configuration School School Public School Electron configuration 1s 2 2s 2 2p 2. Use the periodic table to determine the number of valence electrons in an atom of a certain element. Electrons and the periodic table part. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Electrons and the periodic table part 3a: The bohr model of an. Electron Configuration School School Public School.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write Electron Configuration School School Public School The bohr model of an atom. For example, the electron configuration of lithium,. Use the periodic table to determine the number of valence electrons in an atom of a certain element. Electrons and the periodic table part. Following the 2s sublevel is the 2p, and p sublevels always. Shorthand electron configuration full electron configuration electron shell arrangement; Electrons and the. Electron Configuration School School Public School.
From www.studocu.com
CHM113 T4 Ch7 Worksheets KEY Resource 1 Electron Configuration of Electron Configuration School School Public School Electrons and the periodic table part 3a: For example, the electron configuration of lithium,. Following the 2s sublevel is the 2p, and p sublevels always. The shorthand electron configuration for fe is [ar]4s 2 3d 6. The bohr model of an atom. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Electron configuration. Electron Configuration School School Public School.
From edu.rsc.org
How to teach electron configurations Poster RSC Education Electron Configuration School School Public School Electrons and the periodic table part 3a: Electron configurations describe where electrons are located around the nucleus of an atom. Electron configuration 1s 2 2s 2 2p 2. The shorthand electron configuration for fe is [ar]4s 2 3d 6. Electron configuration of hydrogen (h) 1s 1:. For example, the electron configuration of lithium,. The bohr model of an atom defines. Electron Configuration School School Public School.
From ar.inspiredpencil.com
Printable Periodic Table Of Elements With Electron Configuration Electron Configuration School School Public School Use the periodic table to determine the number of valence electrons in an atom of a certain element. For example, the electron configuration of lithium,. Electron configurations describe where electrons are located around the nucleus of an atom. Write the element symbol and place the dots (valence electrons) on. Following the 2s sublevel is the 2p, and p sublevels always.. Electron Configuration School School Public School.
From stantscience.weebly.com
Electron Configuration High School Biology and Chemistry Electron Configuration School School Public School The bohr model of an atom. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. For example, the electron configuration of lithium,. Electrons and the periodic table part. The shorthand electron configuration for fe is [ar]4s 2 3d 6. Electron configuration of hydrogen (h) 1s 1:. Following the 2s sublevel is the 2p,. Electron Configuration School School Public School.
From www.pinterest.com.mx
Electron Configuration Chart for the Elements Chemistry lessons Electron Configuration School School Public School For example, the electron configuration of lithium,. Electron configurations describe where electrons are located around the nucleus of an atom. Electrons and the periodic table part. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Electron configuration of hydrogen (h) 1s 1:. Use the periodic table to determine the number of valence electrons. Electron Configuration School School Public School.
From studylib.net
Electron Configuration Warren County Schools Electron Configuration School School Public School Following the 2s sublevel is the 2p, and p sublevels always. The bohr model of an atom. Write the element symbol and place the dots (valence electrons) on. The shorthand electron configuration for fe is [ar]4s 2 3d 6. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that. Electron Configuration School School Public School.
From www.pinterest.com
Electron Configurations A Must Know Hack in 2021 Electron Electron Configuration School School Public School Electrons and the periodic table part. Shorthand electron configuration full electron configuration electron shell arrangement; Following the 2s sublevel is the 2p, and p sublevels always. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Electron configuration 1s 2 2s 2 2p 2. Write the element symbol and place the dots (valence electrons). Electron Configuration School School Public School.
From www.pdffiller.com
Electron Configuration Worksheet Moore Public Schools Doc Template Electron Configuration School School Public School Write the element symbol and place the dots (valence electrons) on. Electron configurations describe where electrons are located around the nucleus of an atom. Electrons and the periodic table part 3a: For example, the electron configuration of lithium,. The shorthand electron configuration for fe is [ar]4s 2 3d 6. Shorthand electron configuration full electron configuration electron shell arrangement; Use the. Electron Configuration School School Public School.
From studylib.net
Electron Configurations Effingham County Schools Electron Configuration School School Public School Electron configuration of hydrogen (h) 1s 1:. Electrons and the periodic table part. Electron configurations describe where electrons are located around the nucleus of an atom. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively. Electron Configuration School School Public School.
From iteachly.com
Electron Configuration Worksheet ⋆ Electron Configuration School School Public School Electron configuration of hydrogen (h) 1s 1:. Shorthand electron configuration full electron configuration electron shell arrangement; The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. Write the element symbol and place the dots (valence electrons) on. Use the periodic table to determine the. Electron Configuration School School Public School.
From www.pinterest.com
See the Electron Configuration Diagrams for Atoms of the Elements Electron Configuration School School Public School The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Electron configuration 1s 2 2s 2 2p 2. For example, the electron configuration of lithium,. Use the periodic table to determine the number of valence electrons in an atom of a certain element. The bohr model of an atom defines an atom as a. Electron Configuration School School Public School.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Electron Configuration School School Public School Electrons and the periodic table part. Electron configurations describe where electrons are located around the nucleus of an atom. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. The bohr model of an atom. Electron configuration 1s 2 2s 2 2p 2. Shorthand. Electron Configuration School School Public School.
From studylib.net
Electron Configurations Madison County Schools Electron Configuration School School Public School The shorthand electron configuration for fe is [ar]4s 2 3d 6. Electron configuration 1s 2 2s 2 2p 2. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on. Electron Configuration School School Public School.
From www.rapidlearningcenter.com
High School Chemistry Electron Configuration Electron Configuration School School Public School Electron configurations describe where electrons are located around the nucleus of an atom. Electron configuration of hydrogen (h) 1s 1:. The shorthand electron configuration for fe is [ar]4s 2 3d 6. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. For example, the. Electron Configuration School School Public School.
From studylib.net
Practice Problems (Chapter 8) Electron Configuration Electron Configuration School School Public School Electron configuration 1s 2 2s 2 2p 2. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. Electron configuration of hydrogen (h) 1s 1:. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Electron configurations describe. Electron Configuration School School Public School.
From www.chemistrylearner.com
Free Printable Electron Configuration Worksheets Electron Configuration School School Public School Electron configurations describe where electrons are located around the nucleus of an atom. Electron configuration 1s 2 2s 2 2p 2. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. The bohr model of an atom. The shorthand electron configuration for fe is. Electron Configuration School School Public School.
From www.youtube.com
Introduction to electron configurations AP Chemistry Khan Academy Electron Configuration School School Public School The bohr model of an atom. The shorthand electron configuration for fe is [ar]4s 2 3d 6. Electrons and the periodic table part 3a: The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. Use the periodic table to determine the number of valence. Electron Configuration School School Public School.
From chemistryforhighschool.blogspot.com
Chemistry for High School Electron Configuration Electron Configuration School School Public School Electrons and the periodic table part 3a: The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. For example, the electron configuration of lithium,. Following the 2s sublevel is the 2p, and p sublevels always. Electron configuration 1s 2 2s 2 2p 2. Electron. Electron Configuration School School Public School.
From www.slideshare.net
Electron configuration Electron Configuration School School Public School Electron configuration of hydrogen (h) 1s 1:. Electrons and the periodic table part 3a: The bohr model of an atom. Write the element symbol and place the dots (valence electrons) on. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Use the periodic table to determine the number of valence electrons in an. Electron Configuration School School Public School.
From www.showme.com
Noble Gas Electron Configurations Electron Configuration, High School Electron Configuration School School Public School The bohr model of an atom. Shorthand electron configuration full electron configuration electron shell arrangement; Use the periodic table to determine the number of valence electrons in an atom of a certain element. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. For example, the electron configuration of lithium,. Electron configuration of hydrogen. Electron Configuration School School Public School.
From www.pinterest.com
chlorine Atom model project, Electron configuration, Atom model Electron Configuration School School Public School Following the 2s sublevel is the 2p, and p sublevels always. Electron configuration 1s 2 2s 2 2p 2. Use the periodic table to determine the number of valence electrons in an atom of a certain element. The shorthand electron configuration for fe is [ar]4s 2 3d 6. Electrons and the periodic table part 3a: For example, the electron configuration. Electron Configuration School School Public School.
From www.pinterest.com
Electron Configuration of Atoms gen chem refresher for organic Electron Configuration School School Public School Electrons and the periodic table part 3a: The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. The shorthand electron configuration for fe is [ar]4s 2 3d 6. Electron configuration 1s 2 2s 2 2p 2. Electron configurations describe where electrons are located around. Electron Configuration School School Public School.
From www.youtube.com
The Periodic Table and Electron Configurations (Part 2) YouTube Electron Configuration School School Public School Electron configurations describe where electrons are located around the nucleus of an atom. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. Write the element symbol and place the dots (valence electrons) on. Electron configuration 1s 2 2s 2 2p 2. For example,. Electron Configuration School School Public School.
From www.pinterest.com
Electron Configuration Part 2 Electron configuration, Organic Electron Configuration School School Public School The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. Electron configuration of hydrogen (h) 1s 1:. Electron configurations describe where electrons are located around the nucleus of an atom. Use the periodic table to determine the number of valence electrons in an atom. Electron Configuration School School Public School.
From www.yumpu.com
Electron Configuration Practice Worksheet Electron Configuration School School Public School Electron configurations describe where electrons are located around the nucleus of an atom. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. The bohr model of an atom. Electron configuration of hydrogen (h) 1s 1:. Following the 2s sublevel is the 2p, and. Electron Configuration School School Public School.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Electron Configuration School School Public School Electrons and the periodic table part. The configuration for pb can be written as [xe]6s 2 4f 14 5d 10 6p 2. Shorthand electron configuration full electron configuration electron shell arrangement; Electron configuration of hydrogen (h) 1s 1:. Electron configuration 1s 2 2s 2 2p 2. Electron configurations describe where electrons are located around the nucleus of an atom. Following. Electron Configuration School School Public School.
From www.pinterest.co.uk
Distance Learning Electron Configuration Task Cards PDF + Digital Electron Configuration School School Public School Write the element symbol and place the dots (valence electrons) on. Electron configuration 1s 2 2s 2 2p 2. The shorthand electron configuration for fe is [ar]4s 2 3d 6. Electron configurations describe where electrons are located around the nucleus of an atom. Electrons and the periodic table part. Electrons and the periodic table part 3a: Electron configuration of hydrogen. Electron Configuration School School Public School.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write Electron Configuration School School Public School Shorthand electron configuration full electron configuration electron shell arrangement; Write the element symbol and place the dots (valence electrons) on. Electron configuration 1s 2 2s 2 2p 2. The bohr model of an atom defines an atom as a small, positively charged nucleus surrounded by negatively charged electrons that orbit the nucleus on shells. The configuration for pb can be. Electron Configuration School School Public School.