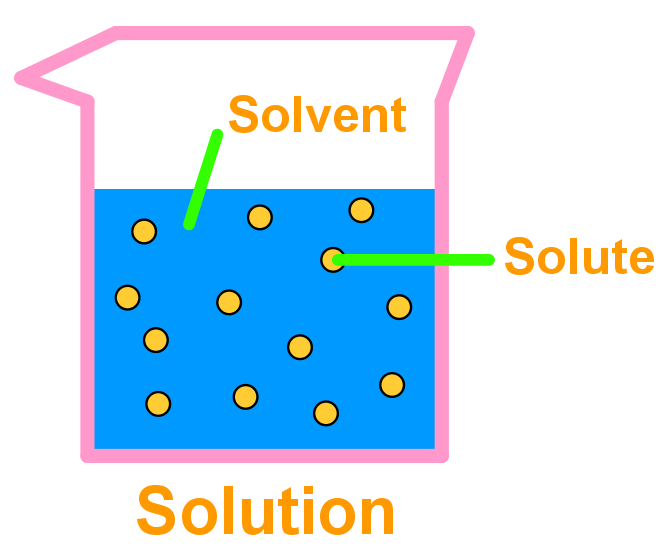
Example Of Solid Solvent Solution . For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. That is, both solute and solvent can be recovered in chemically. Typically, one of the components is present in a. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. The focus of chapter 15: Water was water's role in the formation of aqueous solutions. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed.
from www.studypug.com
Water was water's role in the formation of aqueous solutions. The focus of chapter 15: That is, both solute and solvent can be recovered in chemically. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. Typically, one of the components is present in a.
Introduction to Solution Chemistry Explore Solubility StudyPug
Example Of Solid Solvent Solution That is, both solute and solvent can be recovered in chemically. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. The focus of chapter 15: Typically, one of the components is present in a. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. Water was water's role in the formation of aqueous solutions. That is, both solute and solvent can be recovered in chemically.
From www.slideshare.net
1 5 What Are Solutions Example Of Solid Solvent Solution The formation of a solution from a solute and a solvent is a physical process, not a chemical one. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. Water was water's role in the. Example Of Solid Solvent Solution.
From laboratoryinfo.com
Difference between Solute and Solvent Example Of Solid Solvent Solution Water was water's role in the formation of aqueous solutions. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of. Example Of Solid Solvent Solution.
From www.expii.com
What Is a Solution? — Overview & Examples Expii Example Of Solid Solvent Solution That is, both solute and solvent can be recovered in chemically. The focus of chapter 15: Water was water's role in the formation of aqueous solutions. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of. Example Of Solid Solvent Solution.
From studiousguy.com
8 Solute Examples in Everyday Life StudiousGuy Example Of Solid Solvent Solution Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. That is, both solute and solvent can be recovered in chemically. Water was water's role in the formation of aqueous solutions. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but. Example Of Solid Solvent Solution.
From www.difference101.com
Solute vs. Solvent 5 Key Differences, Pros & Cons, Examples Example Of Solid Solvent Solution Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Typically, one of the components is present in a. The focus of chapter 15: Water was water's role in the formation of aqueous solutions. That. Example Of Solid Solvent Solution.
From brainly.in
Define solute, solvent and solution with example. Brainly.in Example Of Solid Solvent Solution For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. The focus of chapter 15: That is, both solute and solvent can be recovered in chemically. Typically, one of the components is present in a.. Example Of Solid Solvent Solution.
From brainly.ph
Let's Evaluate Complete the table below by giving the solute and the Example Of Solid Solvent Solution For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Water was water's role in the. Example Of Solid Solvent Solution.
From slideplayer.com
Solutions. ppt download Example Of Solid Solvent Solution For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. That is, both solute and solvent can be recovered in chemically. The formation of a solution from a solute and a solvent is a physical. Example Of Solid Solvent Solution.
From courses.lumenlearning.com
The Dissolution Process Chemistry Example Of Solid Solvent Solution Typically, one of the components is present in a. That is, both solute and solvent can be recovered in chemically. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. The focus of chapter 15: For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve. Example Of Solid Solvent Solution.
From sciencenotes.org
What Is an Aqueous Solution? Definition and Examples Example Of Solid Solvent Solution For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. The formation of a solution from a solute and. Example Of Solid Solvent Solution.
From www.studypug.com
Introduction to Solution Chemistry Explore Solubility StudyPug Example Of Solid Solvent Solution That is, both solute and solvent can be recovered in chemically. Typically, one of the components is present in a. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the. Example Of Solid Solvent Solution.
From scienceinfo.com
Solvent Definition, Types, Incredible Uses, Examples Example Of Solid Solvent Solution That is, both solute and solvent can be recovered in chemically. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. The formation of a solution from a solute and a solvent is a physical. Example Of Solid Solvent Solution.
From www.bharatagritech.com
What Are Some Examples Of Solute? Example, 50 OFF Example Of Solid Solvent Solution Water was water's role in the formation of aqueous solutions. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. Typically, one of the components is present in a. Solid solutions are a heterogeneous mixture. Example Of Solid Solvent Solution.
From studylib.net
Solute Solvent Example Example Of Solid Solvent Solution Water was water's role in the formation of aqueous solutions. The focus of chapter 15: The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Typically, one of the components is present in a. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. That. Example Of Solid Solvent Solution.
From devinitionva.blogspot.com
Definition Of Solute Solvent And Solution DEFINITIONVA Example Of Solid Solvent Solution The formation of a solution from a solute and a solvent is a physical process, not a chemical one. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. That is, both solute and solvent. Example Of Solid Solvent Solution.
From www.breakingatom.com
Solubility of Elements and Compounds Example Of Solid Solvent Solution Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. Water was water's role in the formation of aqueous solutions. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will. Example Of Solid Solvent Solution.
From studiousguy.com
8 Solvent Examples in Everyday Life StudiousGuy Example Of Solid Solvent Solution For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. Water was water's role in the formation of aqueous solutions. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or. Example Of Solid Solvent Solution.
From highlands229.blogspot.com
Highlands Middle School 7th Grade Science 20192020 Example Of Solid Solvent Solution Water was water's role in the formation of aqueous solutions. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. The focus of chapter 15: That is, both solute and solvent can be recovered in chemically. Typically, one of the components is present in a. Solid solutions are a heterogeneous mixture. Example Of Solid Solvent Solution.
From www.dreamstime.com
Dissolving Solids. Solubility Chemistry Stock Vector Illustration of Example Of Solid Solvent Solution The focus of chapter 15: Water was water's role in the formation of aqueous solutions. That is, both solute and solvent can be recovered in chemically. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of. Example Of Solid Solvent Solution.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo Example Of Solid Solvent Solution The focus of chapter 15: Water was water's role in the formation of aqueous solutions. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Typically, one of the components is present in a. That is, both solute and solvent can be recovered in chemically. Solid solutions are a heterogeneous mixture. Example Of Solid Solvent Solution.
From www.youtube.com
Solution with examples Identify the solute and solvent YouTube Example Of Solid Solvent Solution The focus of chapter 15: That is, both solute and solvent can be recovered in chemically. Typically, one of the components is present in a. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. The formation of a solution from a solute and a solvent is a physical process, not a chemical one.. Example Of Solid Solvent Solution.
From slideplayer.com
Chemistry of Solutions ppt download Example Of Solid Solvent Solution The focus of chapter 15: That is, both solute and solvent can be recovered in chemically. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. For example, at 25° c and 1 atm pressure,. Example Of Solid Solvent Solution.
From www.youtube.com
Solvent Extraction with Example Chapter 2F.Sc Chemistry Part1 Example Of Solid Solvent Solution Water was water's role in the formation of aqueous solutions. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Typically, one of the components is present in a. The focus of chapter 15: For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in. Example Of Solid Solvent Solution.
From pediaa.com
Difference Between Solvent and Solute Definition, Properties, Examples Example Of Solid Solvent Solution For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. The focus of chapter 15: That is, both solute and solvent can be recovered in chemically. Typically, one of the components is present in a.. Example Of Solid Solvent Solution.
From ar.inspiredpencil.com
Examples Of Gas Solutions Example Of Solid Solvent Solution That is, both solute and solvent can be recovered in chemically. The focus of chapter 15: Water was water's role in the formation of aqueous solutions. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of. Example Of Solid Solvent Solution.
From fr.dreamstime.com
Solution Solide Dans Le Liquide Illustration de Vecteur Illustration Example Of Solid Solvent Solution That is, both solute and solvent can be recovered in chemically. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. Water was water's role in the formation of aqueous solutions. The formation of a. Example Of Solid Solvent Solution.
From study.com
What is a Solvent? Definition & Examples Video & Lesson Transcript Example Of Solid Solvent Solution Typically, one of the components is present in a. Water was water's role in the formation of aqueous solutions. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. The focus of chapter 15: That. Example Of Solid Solvent Solution.
From miraferspatel.blogspot.com
Which Word Describes a Solid That Cannot Dissolve in Water Example Of Solid Solvent Solution Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. That is, both solute and solvent can be recovered. Example Of Solid Solvent Solution.
From www.pinterest.com
Introduction to Solutions Worksheet Set (Solutes, Solvents, and Example Of Solid Solvent Solution Water was water's role in the formation of aqueous solutions. The focus of chapter 15: The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. For example, at 25° c and 1 atm pressure, 20. Example Of Solid Solvent Solution.
From www.slideserve.com
PPT Revision PowerPoint Presentation, free download ID5491285 Example Of Solid Solvent Solution The focus of chapter 15: The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. That is, both solute and solvent can be recovered in chemically. Water was water's role in the formation of aqueous. Example Of Solid Solvent Solution.
From www.youtube.com
Examples of Solute, Solvent and Solution 5 Examples of Solute Solvent Example Of Solid Solvent Solution Typically, one of the components is present in a. That is, both solute and solvent can be recovered in chemically. The focus of chapter 15: For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine.. Example Of Solid Solvent Solution.
From sciencenotes.org
What Is a Solvent? Definition and Examples Example Of Solid Solvent Solution The formation of a solution from a solute and a solvent is a physical process, not a chemical one. The focus of chapter 15: Water was water's role in the formation of aqueous solutions. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity. Example Of Solid Solvent Solution.
From www.youtube.com
Year 7 Science Lesson Solute, Solvent, Solution EdPlace YouTube Example Of Solid Solvent Solution Water was water's role in the formation of aqueous solutions. That is, both solute and solvent can be recovered in chemically. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. The focus of chapter 15: For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will. Example Of Solid Solvent Solution.
From www.vrogue.co
Solvent Definition Types Incredible Uses Examples vrogue.co Example Of Solid Solvent Solution Water was water's role in the formation of aqueous solutions. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or. Example Of Solid Solvent Solution.
From www.studypug.com
Introduction to Solution Chemistry Explore Solubility StudyPug Example Of Solid Solvent Solution The focus of chapter 15: Typically, one of the components is present in a. Water was water's role in the formation of aqueous solutions. For example, at 25° c and 1 atm pressure, 20 g of iodine crystals will dissolve in 100 ml of ethyl alcohol, but the same quantity of water will dissolve only 0.30 g of iodine. Solid. Example Of Solid Solvent Solution.