Chlorine Fluorine Atom . Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Learn about their properties, uses, and biological. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Some metals, such as nickel, are. Learn the names, symbols, and properties of the seven diatomic. This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. This makes the fluoride anion so. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. The halogen elements are group 17 on the periodic table. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together.
from mavink.com
Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. This makes the fluoride anion so. Learn the names, symbols, and properties of the seven diatomic. This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Some metals, such as nickel, are. The halogen elements are group 17 on the periodic table.
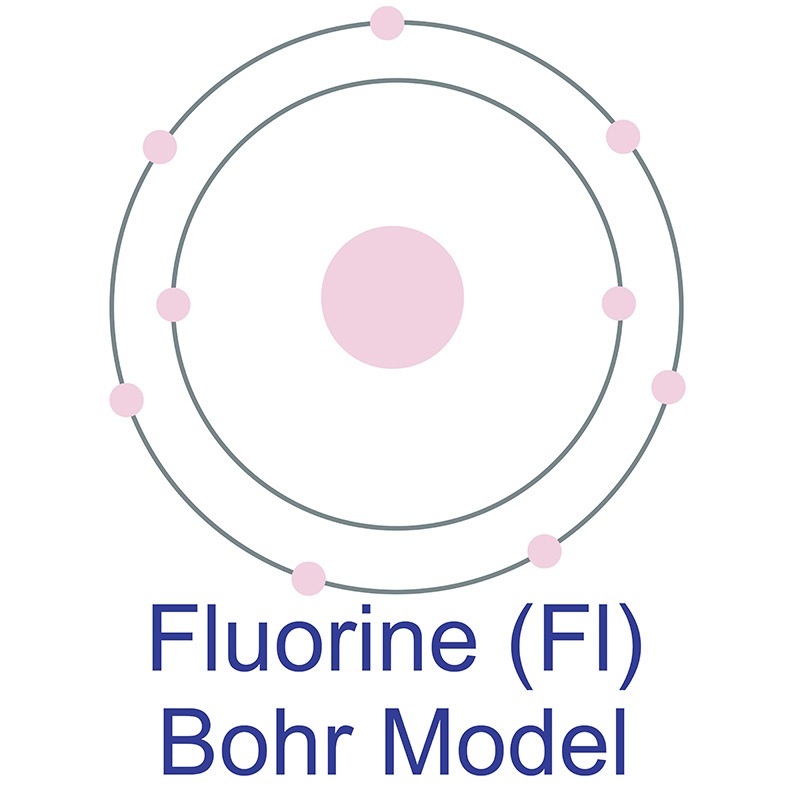
Fluorine Atom Diagram
Chlorine Fluorine Atom Learn about their properties, uses, and biological. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Learn about their properties, uses, and biological. The halogen elements are group 17 on the periodic table. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Learn the names, symbols, and properties of the seven diatomic. Some metals, such as nickel, are. This makes the fluoride anion so. This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine.
From imgbin.com
Nitrogen Trifluoride Chlorine Trifluoride Fluorine PNG, Clipart Chlorine Fluorine Atom Learn the names, symbols, and properties of the seven diatomic. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Learn about their properties, uses, and biological. The halogen elements are group. Chlorine Fluorine Atom.
From www.toppr.com
Chlorine atom in its third excited state with fluo Chlorine Fluorine Atom This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Some metals, such as nickel, are. Diatomic elements are pure elements that form molecules consisting. Chlorine Fluorine Atom.
From depositphotos.com
Fluorine Chemical Element Chemical Symbol Atomic Number Atomic Mass Chlorine Fluorine Atom The halogen elements are group 17 on the periodic table. This makes the fluoride anion so. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic. Chlorine Fluorine Atom.
From pnghut.com
Thiazyl Trifluoride Fluoride Chlorine Tetrasulfur Tetranitride Yellow Chlorine Fluorine Atom Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. The halogen elements are group 17 on the periodic table. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down. Chlorine Fluorine Atom.
From owlcation.com
Fluorine The Most Reactive Element in the Periodic Table Owlcation Chlorine Fluorine Atom Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. The halogen elements are group 17 on the periodic table. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Learn about their properties, uses, and biological. Learn the names, symbols, and properties of the seven diatomic. Some metals, such as nickel, are. This article contains. Chlorine Fluorine Atom.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Chlorine Fluorine Atom The halogen elements are group 17 on the periodic table. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. This article contains. Chlorine Fluorine Atom.
From valenceelectrons.com
Fluorine Electron Configuration With Full Orbital Diagram Chlorine Fluorine Atom The halogen elements are group 17 on the periodic table. Learn about their properties, uses, and biological. Learn the names, symbols, and properties of the seven diatomic. Some metals, such as nickel, are. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and. Chlorine Fluorine Atom.
From mungfali.com
Fluorine Electron Configuration Diagram Chlorine Fluorine Atom The halogen elements are group 17 on the periodic table. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Learn the names, symbols, and properties of the seven diatomic. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Fluorine (f 2), composed of two fluorine atoms, combines with all. Chlorine Fluorine Atom.
From spmchemistry.blog.onlinetuition.com.my
Covalent Bonding SPM Chemistry Chlorine Fluorine Atom Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Some metals, such as nickel, are. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. This makes the fluoride anion so. This article contains comparison of key thermal and atomic properties. Chlorine Fluorine Atom.
From www.alamy.com
Fluorine molecule, illustration Stock Photo Alamy Chlorine Fluorine Atom Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Learn about their properties, uses, and biological. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. This makes the fluoride anion so.. Chlorine Fluorine Atom.
From www.nuclear-power.com
Fluorine Electron Affinity Electronegativity Ionization Energy of Chlorine Fluorine Atom This makes the fluoride anion so. Some metals, such as nickel, are. This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Learn the names, symbols, and properties of the seven diatomic. Fluorine (f 2), composed of two fluorine. Chlorine Fluorine Atom.
From ajorpng.blogspot.com
Chlorine Pentafluoride Lewis / Chlorine pentafluoride is a square Chlorine Fluorine Atom Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. This makes the fluoride anion so. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Some metals, such as nickel, are. Fluorine, though. Chlorine Fluorine Atom.
From stock.adobe.com
Group 17 (6A) of the Periodic Table of Elements. Fluorine, chlorine Chlorine Fluorine Atom They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. The halogen elements are group 17 on the periodic table. Learn about their properties, uses, and biological. This makes the fluoride anion so. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Learn the names, symbols, and properties of the seven diatomic. Some metals, such. Chlorine Fluorine Atom.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Fluorine Atom Learn the names, symbols, and properties of the seven diatomic. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. This makes the fluoride anion so. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Fluorine, though higher than chlorine in the periodic table, has a very. Chlorine Fluorine Atom.
From guidedatadarren.z21.web.core.windows.net
Bohr Diagram Of A Chlorine Atom Chlorine Fluorine Atom Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Some metals, such as nickel, are. Learn about their properties, uses, and biological. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Learn the names, symbols, and properties of the seven diatomic. This article contains comparison of key thermal and. Chlorine Fluorine Atom.
From en.wikipedia.org
FileChlorinemonofluoride3DvdW.png Wikipedia Chlorine Fluorine Atom Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Learn the names, symbols, and properties of the seven diatomic. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. This makes the fluoride anion so. Learn how the atomic radius, electronegativity, electron. Chlorine Fluorine Atom.
From material-properties.org
Sulfur and Chlorine Comparison Properties Material Properties Chlorine Fluorine Atom Learn about their properties, uses, and biological. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Learn the names, symbols, and properties of the seven diatomic. Fluorine, though higher than chlorine. Chlorine Fluorine Atom.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector Chlorine Fluorine Atom Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Learn the names, symbols, and properties of the seven diatomic. Fluorine, though higher. Chlorine Fluorine Atom.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Fluorine Atom They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Some metals, such as nickel, are. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Learn about their properties, uses, and biological. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides.. Chlorine Fluorine Atom.
From www.shutterstock.com
Atomic Structure Lewis Dot Diagram Fluorine Stock Vector (Royalty Free Chlorine Fluorine Atom Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the. Chlorine Fluorine Atom.
From www.youtube.com
How to Balance F2 + Cl2 = ClF3 (Fluorine gas + Chlorine gas) YouTube Chlorine Fluorine Atom Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. The halogen elements are group 17 on the periodic table. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Learn the names, symbols, and properties of the seven diatomic. They. Chlorine Fluorine Atom.
From www.chemtube3d.com
Molecular orbitals in Fluorine Chlorine Fluorine Atom The halogen elements are group 17 on the periodic table. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Learn about their. Chlorine Fluorine Atom.
From www.dreamstime.com
Fluorine Atom Bohr Model With Proton, Neutron And Electron Stock Chlorine Fluorine Atom This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. The halogen elements are group 17 on the periodic table. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points. Chlorine Fluorine Atom.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Fluorine Atom Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. This makes the fluoride anion so. The halogen elements are group 17 on the periodic table. This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the. Chlorine Fluorine Atom.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Chlorine Fluorine Atom This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Learn. Chlorine Fluorine Atom.
From animalia-life.club
Fluorine Molecule Project Chlorine Fluorine Atom This makes the fluoride anion so. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Some metals, such as nickel, are. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. The halogen elements are group 17 on the periodic table.. Chlorine Fluorine Atom.
From stock.adobe.com
types of atomic radius of a chemical element. Nitrogen, oxygen Chlorine Fluorine Atom They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. This makes the fluoride anion so. The halogen elements are group 17 on the periodic table. Some metals, such as nickel, are. Fluorine, though higher than chlorine in the. Chlorine Fluorine Atom.
From www.examples.com
Fluorine (F) Definition, Preparation, Properties, Uses, Compounds Chlorine Fluorine Atom Some metals, such as nickel, are. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. The halogen elements are group 17 on the periodic table. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic. Chlorine Fluorine Atom.
From www.vectorstock.com
Diagram representation element fluorine Royalty Free Vector Chlorine Fluorine Atom The halogen elements are group 17 on the periodic table. Diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. This makes the fluoride anion so. They are fluorine, chlorine, bromine, iodine,. Chlorine Fluorine Atom.
From valenceelectrons.com
Fluorine(F) electron configuration and orbital diagram Chlorine Fluorine Atom This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. This makes the fluoride anion so. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine, chlorine, bromine and iodine vary down the group 7. Learn about their properties, uses, and biological. The. Chlorine Fluorine Atom.
From utedzz.blogspot.com
Periodic Table Fluorine Picture Periodic Table Timeline Chlorine Fluorine Atom Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Learn the names, symbols, and properties of the seven diatomic. The halogen elements are group 17 on the periodic table. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Some metals, such as nickel, are. Learn about their properties, uses, and biological. Fluorine (f 2),. Chlorine Fluorine Atom.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Fluorine Atom Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Some metals, such as nickel, are. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable. Chlorine Fluorine Atom.
From www.alamy.com
Chlorine atomic structure Cut Out Stock Images & Pictures Alamy Chlorine Fluorine Atom The halogen elements are group 17 on the periodic table. This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form. Chlorine Fluorine Atom.
From mavink.com
Fluorine Atom Diagram Chlorine Fluorine Atom Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Learn the names, symbols, and properties of the seven diatomic. This article contains comparison of key thermal and atomic properties of fluorine and chlorine, two comparable chemical elements from the periodic table. Diatomic elements are pure elements. Chlorine Fluorine Atom.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Fluorine Atom Fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent fluorides. Learn about their properties, uses, and biological. This makes the fluoride anion so. They are fluorine, chlorine, bromine, iodine, astatine, and tennessine. Learn how the atomic radius, electronegativity, electron affinity, and melting and boiling points of fluorine,. Chlorine Fluorine Atom.