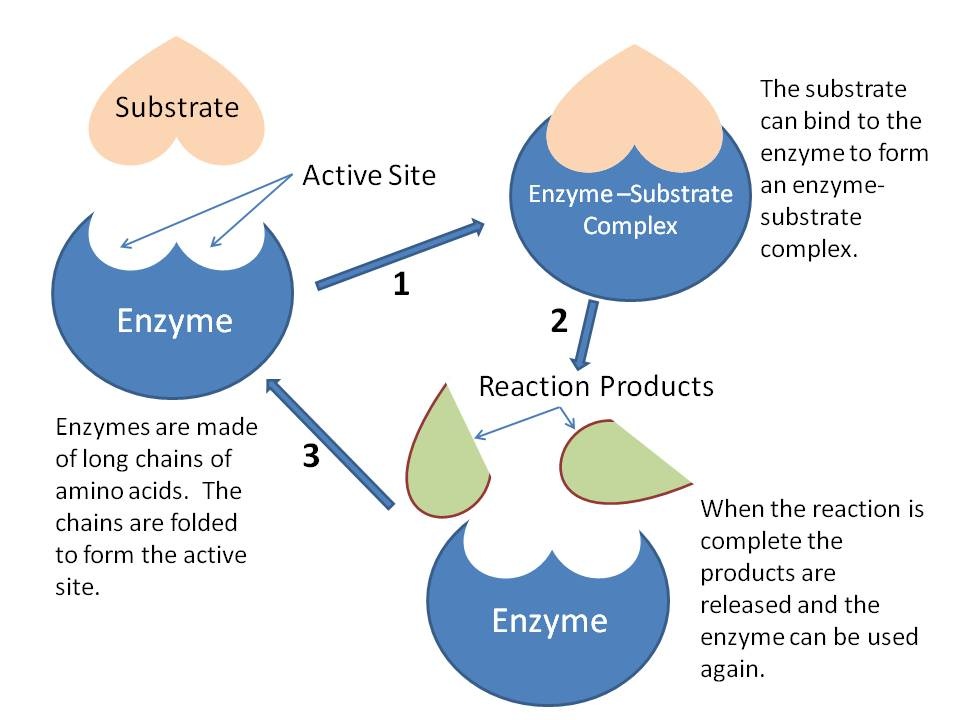
Do Enzymes Have Hydrogen Bonds . They may bond either temporarily through ionic or hydrogen bonds, or permanently. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. Learn about their components, properties, structure, and how they work to. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong.
from biology4ibdp.weebly.com
Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Learn about their components, properties, structure, and how they work to. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph.
2.5 Enzymes BIOLOGY4IBDP
Do Enzymes Have Hydrogen Bonds Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Learn about their components, properties, structure, and how they work to.
From gohalfsies.com
Enzymology Threedimensional structure of enzymes Do Enzymes Have Hydrogen Bonds Learn about their components, properties, structure, and how they work to. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in. Do Enzymes Have Hydrogen Bonds.
From www.almrsal.com
العوامل المؤثرة على الإنزيمات المرسال Do Enzymes Have Hydrogen Bonds The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. Hydrogen bonds, ionic bonds and. Do Enzymes Have Hydrogen Bonds.
From ibiologia.com
Enzymes Definition, Classification & Functions Do Enzymes Have Hydrogen Bonds They may bond either temporarily through ionic or hydrogen bonds, or permanently. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. Learn about their components, properties, structure, and how they work to. Enzymes are. Do Enzymes Have Hydrogen Bonds.
From mammothmemory.net
Example of how protease enzymes break down proteins Do Enzymes Have Hydrogen Bonds It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Learn about their components, properties, structure, and how they work to. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. The substrate binds. Do Enzymes Have Hydrogen Bonds.
From www.slideserve.com
PPT ENZYMES PowerPoint Presentation, free download ID1117093 Do Enzymes Have Hydrogen Bonds Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Enzymes are protein. Do Enzymes Have Hydrogen Bonds.
From projectopenletter.com
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates Do Enzymes Have Hydrogen Bonds The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. Learn about their components, properties, structure, and how they work to. They may bond either temporarily through ionic or hydrogen bonds, or permanently. It has been proposed that some remarkable enzymic. Do Enzymes Have Hydrogen Bonds.
From www.slideserve.com
PPT HL Chemistry Option B Human Biochemistry PowerPoint Do Enzymes Have Hydrogen Bonds Learn about their components, properties, structure, and how they work to. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of. Do Enzymes Have Hydrogen Bonds.
From guides.hostos.cuny.edu
Chapter 9 Proteins and Enzymes CHE 120 Introduction to Organic Do Enzymes Have Hydrogen Bonds It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. They may bond either temporarily through. Do Enzymes Have Hydrogen Bonds.
From joioycgbl.blob.core.windows.net
Types Of Hydrogen Bonds at Luis Newell blog Do Enzymes Have Hydrogen Bonds Learn about their components, properties, structure, and how they work to. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges. Do Enzymes Have Hydrogen Bonds.
From stock.adobe.com
Biological diagram show mechanism of enzyme substrate interaction by Do Enzymes Have Hydrogen Bonds Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. Learn about their components, properties, structure, and how they work to. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of. Do Enzymes Have Hydrogen Bonds.
From joiqqqtcc.blob.core.windows.net
Enzymes In Biology A Level at Gary Velez blog Do Enzymes Have Hydrogen Bonds Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. They may bond either temporarily through ionic or hydrogen bonds, or permanently. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of. Do Enzymes Have Hydrogen Bonds.
From www.youtube.com
How many hydrogen bonds a water molecule can form Hydrogen Bonding in Do Enzymes Have Hydrogen Bonds It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. Learn about their components, properties, structure, and how they work to. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in. Do Enzymes Have Hydrogen Bonds.
From exyjskkyy.blob.core.windows.net
What Enzyme Breaks Down Hydrogen Bonds at Bobby Wilkerson blog Do Enzymes Have Hydrogen Bonds Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. They may bond either temporarily through ionic or. Do Enzymes Have Hydrogen Bonds.
From ffden-2.phys.uaf.edu
Soap and Water Interaction Do Enzymes Have Hydrogen Bonds Learn about their components, properties, structure, and how they work to. They may bond either temporarily through ionic or hydrogen bonds, or permanently. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. The substrate binds. Do Enzymes Have Hydrogen Bonds.
From www.savemyexams.com
Nucleic Acid Structure & Function SL IB Biology Revision Notes 2025 Do Enzymes Have Hydrogen Bonds Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in. Do Enzymes Have Hydrogen Bonds.
From sites.google.com
Biochemistry WHS Biology Do Enzymes Have Hydrogen Bonds Learn about their components, properties, structure, and how they work to. They may bond either temporarily through ionic or hydrogen bonds, or permanently. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. Hydrogen bonds provide most of the directional interactions. Do Enzymes Have Hydrogen Bonds.
From www.nagwa.com
Question Video Identifying Which Compound Could Form Hydrogen Bonds Do Enzymes Have Hydrogen Bonds Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Enzymes are protein. Do Enzymes Have Hydrogen Bonds.
From www.researchgate.net
Hydrogen bond interactions of the substrate in the active site of the Do Enzymes Have Hydrogen Bonds The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Learn about their components,. Do Enzymes Have Hydrogen Bonds.
From abettes-culinary.com
How Do Hydrogen Bonds Compare With Other Intermolecular Forces Apex Do Enzymes Have Hydrogen Bonds It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. They may bond either temporarily. Do Enzymes Have Hydrogen Bonds.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 Do Enzymes Have Hydrogen Bonds Learn about their components, properties, structure, and how they work to. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. The article reviews the evidence and arguments for the. Do Enzymes Have Hydrogen Bonds.
From courses.lumenlearning.com
Reading Protein Structure Biology I Do Enzymes Have Hydrogen Bonds Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. They may bond either temporarily through ionic or hydrogen bonds, or permanently. It has been proposed that some remarkable enzymic catalytic effects. Do Enzymes Have Hydrogen Bonds.
From biology4ibdp.weebly.com
2.5 Enzymes BIOLOGY4IBDP Do Enzymes Have Hydrogen Bonds They may bond either temporarily through ionic or hydrogen bonds, or permanently. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. Learn about their components, properties, structure, and how they work to. The article reviews the evidence and. Do Enzymes Have Hydrogen Bonds.
From saylordotorg.github.io
Amino Acids, Proteins, and Enzymes Do Enzymes Have Hydrogen Bonds The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Learn about their components,. Do Enzymes Have Hydrogen Bonds.
From joiudcrpy.blob.core.windows.net
What Chemical Reactions Do Enzymes Catalyze at Javier Hodge blog Do Enzymes Have Hydrogen Bonds The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. Learn about their components, properties, structure, and how they work to. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. Enzymes are protein macromolecules that. Do Enzymes Have Hydrogen Bonds.
From joiulfeej.blob.core.windows.net
Does Dried Pineapple Still Have Enzymes at Nancy Cooper blog Do Enzymes Have Hydrogen Bonds It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions. Do Enzymes Have Hydrogen Bonds.
From www.lecturio.com
Basics of Enzymes Concise Medical Knowledge Do Enzymes Have Hydrogen Bonds Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. The. Do Enzymes Have Hydrogen Bonds.
From www.expii.com
Enzyme Inhibition — Overview & Types Expii Do Enzymes Have Hydrogen Bonds The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. They may bond either temporarily through ionic or. Do Enzymes Have Hydrogen Bonds.
From www.researchgate.net
Chemical structures of DNA Hydrogen bonds shown as dotted lines (69 Do Enzymes Have Hydrogen Bonds Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. Learn about their components, properties, structure, and how they work to. Enzymes are protein macromolecules that act as biological catalysts. Do Enzymes Have Hydrogen Bonds.
From saylordotorg.github.io
Amino Acids, Proteins, and Enzymes Do Enzymes Have Hydrogen Bonds It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Learn about their components, properties, structure, and how they work to. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Hydrogen bonds provide most. Do Enzymes Have Hydrogen Bonds.
From www.wikidoc.org
DNA wikidoc Do Enzymes Have Hydrogen Bonds The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. Learn about their components, properties, structure, and how they work to. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living. Do Enzymes Have Hydrogen Bonds.
From www.biologyexams4u.com
What are the 6 Major Chemical Bonds or Interactions In Proteins? Do Enzymes Have Hydrogen Bonds The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein. Do Enzymes Have Hydrogen Bonds.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Do Enzymes Have Hydrogen Bonds Learn about their components, properties, structure, and how they work to. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be. Do Enzymes Have Hydrogen Bonds.
From mccnsulting.web.fc2.com
How does catalase break down hydrogen peroxide? Do Enzymes Have Hydrogen Bonds Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Enzymes are protein macromolecules that act as biological catalysts for biochemical reactions in living systems. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence. Do Enzymes Have Hydrogen Bonds.
From stock.adobe.com
Vetor de hydrogen bond. chemistry lesson. Infographic. hydrogen bonds Do Enzymes Have Hydrogen Bonds The article reviews the evidence and arguments for the importance of short, strong hydrogen bonds in enzyme active sites and their. Hydrogen bonds provide most of the directional interactions that underpin protein folding, protein structure and molecular recognition. They may bond either temporarily through ionic or hydrogen bonds, or permanently. Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and. Do Enzymes Have Hydrogen Bonds.
From joioycgbl.blob.core.windows.net
Types Of Hydrogen Bonds at Luis Newell blog Do Enzymes Have Hydrogen Bonds Hydrogen bonds, ionic bonds and covalent bonds (disulfide bridges and peptide bonds) can all be disrupted by large changes in temperate and ph. Learn about their components, properties, structure, and how they work to. It has been proposed that some remarkable enzymic catalytic effects can be explained by the existence of unusually strong. They may bond either temporarily through ionic. Do Enzymes Have Hydrogen Bonds.