Single Use Medical Devices Policy . The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies.
from www.jointcommission.org
The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies.
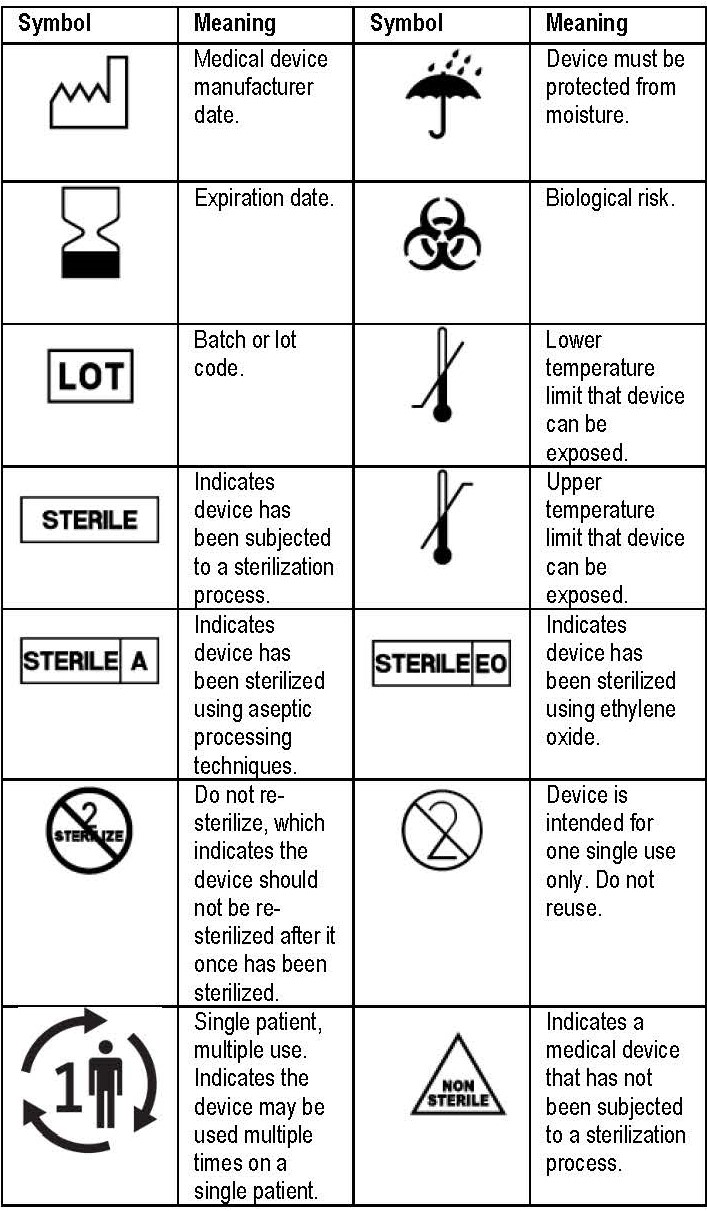
Symbol table for device labeling
Single Use Medical Devices Policy Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies.
From www.slideserve.com
PPT Single Use Medical Devices Reprocessing Market PowerPoint Single Use Medical Devices Policy The european commission (ec) and the medicines and. Technical / quality issues with original products of certain companies. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Single Use Medical Devices Policy.
From blog.lighthouseoptics.com
Lighthouse InSight Why Consider Singleuse Medical Devices? Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.youtube.com
Guidance for Cleaning, Disinfection and Sterilization of Reusable Single Use Medical Devices Policy Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Single Use Medical Devices Policy.
From www.mordorintelligence.com
AsiaPacific Singleuse Medical Device Reprocessing Market Size Single Use Medical Devices Policy Technical / quality issues with original products of certain companies. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.ukwebwire.com
Navigating The Evolution SingleUse Medical Device Reprocessing Market Single Use Medical Devices Policy Technical / quality issues with original products of certain companies. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From issuu.com
SingleUse Medical Device Reprocessing Market Industry Analysis, Size Single Use Medical Devices Policy The european commission (ec) and the medicines and. Technical / quality issues with original products of certain companies. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Single Use Medical Devices Policy.
From coremedsurgical.com
Single Use Medical Device (SUD) Reprocessing Single Use Medical Devices Policy The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From emmainternational.com
EU MDR Ready Reprocessed Single Use Devices Single Use Medical Devices Policy Technical / quality issues with original products of certain companies. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From dokumen.tips
(PDF) Singleuse medical devices implications and … · Singleuse Single Use Medical Devices Policy The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From www.slideserve.com
PPT Single Use Medical Devices Reprocessing Market PowerPoint Single Use Medical Devices Policy The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From hospitalsmagazine.com
Singleuse medical devices HOSPITALS MAGAZINE Single Use Medical Devices Policy Technical / quality issues with original products of certain companies. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.imisite.org
Global Study Report on SingleUse Medical Device Reprocessing Market Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. The european commission (ec) and the medicines and. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From markwideresearch.com
MEA Singleuse Medical Device Reprocessing Market 20242032 Size Single Use Medical Devices Policy The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From www.einpresswire.com
Reprocessing and Reuse of Single Use Medical Devices Market Current and Single Use Medical Devices Policy The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From www.slideserve.com
PPT 1. Global Single Use Medical Devices Reprocessin PowerPoint Single Use Medical Devices Policy The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From dokumen.tips
(PDF) Reprocessing Singleuse Medical Devices DOKUMEN.TIPS Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.researchgate.net
(PDF) Green Servitization in the SingleUse Medical Device Industry Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.jointcommission.org
Symbol table for device labeling Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. The european commission (ec) and the medicines and. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From www.dreamstime.com
Singleuse Medical Devices Symbol. Concept of Packaging Stock Vector Single Use Medical Devices Policy The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From www.hukuibio.com
Singleuse Medical Devices Are Transforming the Industry Hukui Single Use Medical Devices Policy The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From coremedsurgical.com
Can a Single Use Medical Device Be Used More Than Once? https Single Use Medical Devices Policy The european commission (ec) and the medicines and. Technical / quality issues with original products of certain companies. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Single Use Medical Devices Policy.
From www.ibottles.net
Single Use Medical Device Innovative Bottles Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From angelanjohnson.com
Medical Devices Angela N Johnson Single Use Medical Devices Policy Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Single Use Medical Devices Policy.
From studylib.net
Medical Devices Management Policy V7.0 Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. The european commission (ec) and the medicines and. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From medical-technology.nridigital.com
Will Covid19 bring a return to singleuse medical devices? Medical Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.youtube.com
What are medical devices? YouTube Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.databridgemarketresearch.com
AsiaPacific Single Use Medical Devices Reprocessing Market Scope Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.slideserve.com
PPT 1. Global Single Use Medical Devices Reprocessing PowerPoint Single Use Medical Devices Policy The european commission (ec) and the medicines and. Technical / quality issues with original products of certain companies. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Single Use Medical Devices Policy.
From www.mdpi.com
Sustainability Free FullText Green Servitization in the SingleUse Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.databridgemarketresearch.com
Global Single Use Medical Devices Reprocessing Market Research Report Single Use Medical Devices Policy The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. Single Use Medical Devices Policy.
From www.rdhmag.com
SINGLEUSE (DISPOSABLE) DEVICES Registered Dental Hygienist (RDH Single Use Medical Devices Policy Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Single Use Medical Devices Policy.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.slideserve.com
PPT Reprocessing SingleUse Medical Devices with Plasma PowerPoint Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From biomedhealthcare.wordpress.com
single use vs reusable use BioMed Healthcare Single Use Medical Devices Policy The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Technical / quality issues with original products of certain companies. The european commission (ec) and the medicines and. Single Use Medical Devices Policy.
From www.researchandmarkets.com
Singleuse Medical Device Reprocessing Market Growths, Trends, and Single Use Medical Devices Policy The european commission (ec) and the medicines and. Technical / quality issues with original products of certain companies. The responsibility of designating a medical device as single use or single patient use lies with the manufacturer and this must be clearly stated on. Single Use Medical Devices Policy.