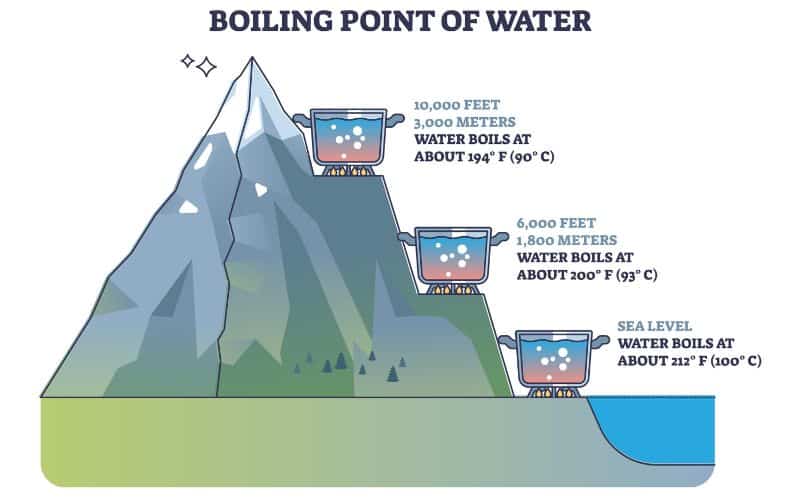
Does It Take Longer To Boil Water In High Altitude . It depends on where you’re doing the. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Less energy means less heat, which means water will boil at a lower. As the altitude increases, the atmospheric pressure. The temperature at which water boils decreases as elevation increases. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. More energy, as in higher heat, makes molecules move even faster. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. It seems like one of those basic science facts: Because foods that are boiled are.
from www.myopencountry.com
Less energy means less heat, which means water will boil at a lower. As the altitude increases, the atmospheric pressure. More energy, as in higher heat, makes molecules move even faster. It seems like one of those basic science facts: Water boils at 212 degrees fahrenheit (100 degrees celsius), right? At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. It depends on where you’re doing the. The temperature at which water boils decreases as elevation increases. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water.
Boiling Water at Higher Altitude What You Need to Know My Open Country
Does It Take Longer To Boil Water In High Altitude At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. It seems like one of those basic science facts: At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. It depends on where you’re doing the. More energy, as in higher heat, makes molecules move even faster. Because foods that are boiled are. The temperature at which water boils decreases as elevation increases. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. Less energy means less heat, which means water will boil at a lower. As the altitude increases, the atmospheric pressure.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does It Take Longer To Boil Water In High Altitude More energy, as in higher heat, makes molecules move even faster. It depends on where you’re doing the. Less energy means less heat, which means water will boil at a lower. Because foods that are boiled are. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? As the altitude increases, the atmospheric pressure. The temperature at which water boils. Does It Take Longer To Boil Water In High Altitude.
From www.numerade.com
SOLVEDGENERAL Boiling Point At higher altitudes, water boils at lower Does It Take Longer To Boil Water In High Altitude As the altitude increases, the atmospheric pressure. It depends on where you’re doing the. Because foods that are boiled are. It seems like one of those basic science facts: When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. When you boil water, you're literally speeding up liquid. Does It Take Longer To Boil Water In High Altitude.
From www.youtube.com
Cooking at high altitude Baking at higher altitude Boiling water at Does It Take Longer To Boil Water In High Altitude Water boils at 212 degrees fahrenheit (100 degrees celsius), right? Less energy means less heat, which means water will boil at a lower. As the altitude increases, the atmospheric pressure. Because foods that are boiled are. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. At a. Does It Take Longer To Boil Water In High Altitude.
From www.youtube.com
Why does water boils faster at higher altitudes ? In English YouTube Does It Take Longer To Boil Water In High Altitude As the altitude increases, the atmospheric pressure. It seems like one of those basic science facts: When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. The temperature at which water boils decreases as elevation increases. Less energy means less heat, which means water will boil at a lower. When atmospheric pressure is. Does It Take Longer To Boil Water In High Altitude.
From slideplayer.com
Hope You Enjoyed Your Break!! ppt download Does It Take Longer To Boil Water In High Altitude Water boils at 212 degrees fahrenheit (100 degrees celsius), right? When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Less energy means less heat, which means water will boil at a lower.. Does It Take Longer To Boil Water In High Altitude.
From cookthink.com
How Long Does it Take for Water to Boil CookThink Does It Take Longer To Boil Water In High Altitude At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. The temperature at which water boils decreases as elevation increases. Because foods that are boiled are. It depends on where you’re doing the. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to. Does It Take Longer To Boil Water In High Altitude.
From deepstash.com
What is the boiling point of water at the top of Mount Everest? Deepstash Does It Take Longer To Boil Water In High Altitude Water boils at 212 degrees fahrenheit (100 degrees celsius), right? Because foods that are boiled are. Less energy means less heat, which means water will boil at a lower. As the altitude increases, the atmospheric pressure. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. One of the most significant changes that. Does It Take Longer To Boil Water In High Altitude.
From thecookwaregeek.com
How Long Does it Take to Boil Water? The Cookware Geek Does It Take Longer To Boil Water In High Altitude More energy, as in higher heat, makes molecules move even faster. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Less energy means less heat, which means water will boil. Does It Take Longer To Boil Water In High Altitude.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does It Take Longer To Boil Water In High Altitude Water boils at 212 degrees fahrenheit (100 degrees celsius), right? As the altitude increases, the atmospheric pressure. The temperature at which water boils decreases as elevation increases. Less energy means less heat, which means water will boil at a lower. More energy, as in higher heat, makes molecules move even faster. Because foods that are boiled are. It depends on. Does It Take Longer To Boil Water In High Altitude.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does It Take Longer To Boil Water In High Altitude One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. The temperature at which water boils decreases as elevation increases. More energy, as in higher heat, makes molecules move even faster. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? It seems like one of those basic science facts:. Does It Take Longer To Boil Water In High Altitude.
From fyozkayiz.blob.core.windows.net
Why Does Water Boil At 100 Degrees Celsius At Sea Level at Jesus Newman Does It Take Longer To Boil Water In High Altitude It seems like one of those basic science facts: The temperature at which water boils decreases as elevation increases. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a. Does It Take Longer To Boil Water In High Altitude.
From www.thespruceeats.com
The Boiling Point of Water at Various Altitudes Does It Take Longer To Boil Water In High Altitude One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. It seems like one of those basic science facts: Water boils at 212 degrees fahrenheit (100 degrees celsius),. Does It Take Longer To Boil Water In High Altitude.
From us.btechshala.com
Best Steps on How Long Does Water Take to Boil in 2024 B.Techshala Does It Take Longer To Boil Water In High Altitude More energy, as in higher heat, makes molecules move even faster. Because foods that are boiled are. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. It depends on where you’re doing the. One of the most significant changes that occur in high altitude areas concerning cooking. Does It Take Longer To Boil Water In High Altitude.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly Does It Take Longer To Boil Water In High Altitude Because foods that are boiled are. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Less energy means less heat, which means water will boil at a lower. As the altitude increases, the atmospheric pressure. When you boil. Does It Take Longer To Boil Water In High Altitude.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does It Take Longer To Boil Water In High Altitude As the altitude increases, the atmospheric pressure. More energy, as in higher heat, makes molecules move even faster. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Because foods that are boiled are. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more. Does It Take Longer To Boil Water In High Altitude.
From www.myopencountry.com
Boiling Water at Higher Altitude What You Need to Know My Open Country Does It Take Longer To Boil Water In High Altitude One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. It seems like one of those basic science facts: Water boils at 212 degrees fahrenheit (100 degrees celsius),. Does It Take Longer To Boil Water In High Altitude.
From www.youtube.com
Study Boiling Point of Water with Altitude YouTube Does It Take Longer To Boil Water In High Altitude It depends on where you’re doing the. As the altitude increases, the atmospheric pressure. Less energy means less heat, which means water will boil at a lower. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. One of the most significant changes that occur in high altitude areas. Does It Take Longer To Boil Water In High Altitude.
From btechshala.com
How Long Does it Take to Boil Water? Best Time in 2023 Does It Take Longer To Boil Water In High Altitude One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. It seems like one of those basic science. Does It Take Longer To Boil Water In High Altitude.
From fyoxouusm.blob.core.windows.net
Does It Take Water Longer To Boil In High Altitude at Joseph May blog Does It Take Longer To Boil Water In High Altitude At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. As the altitude increases, the atmospheric pressure. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. Less energy means less heat, which means water will boil at a. Does It Take Longer To Boil Water In High Altitude.
From fyoxouusm.blob.core.windows.net
Does It Take Water Longer To Boil In High Altitude at Joseph May blog Does It Take Longer To Boil Water In High Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Because foods that are boiled are. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Less energy means less heat, which means water will boil at a lower. More energy, as. Does It Take Longer To Boil Water In High Altitude.
From www.wonderopolis.org
Why Does Water Boil Faster at Higher Altitude? Wonderopolis Does It Take Longer To Boil Water In High Altitude When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. It seems like one of those basic science facts: At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. The temperature at which water boils decreases as. Does It Take Longer To Boil Water In High Altitude.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does It Take Longer To Boil Water In High Altitude At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. It seems like one of those basic science facts: Less energy means less heat, which means water will boil at a lower. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? More energy, as in higher heat, makes. Does It Take Longer To Boil Water In High Altitude.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly Does It Take Longer To Boil Water In High Altitude It seems like one of those basic science facts: Because foods that are boiled are. As the altitude increases, the atmospheric pressure. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature.. Does It Take Longer To Boil Water In High Altitude.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does It Take Longer To Boil Water In High Altitude Less energy means less heat, which means water will boil at a lower. It seems like one of those basic science facts: At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? Because foods that are boiled are. As. Does It Take Longer To Boil Water In High Altitude.
From hxemrtltm.blob.core.windows.net
Does Oil Take Longer To Boil at Jerry Scoggins blog Does It Take Longer To Boil Water In High Altitude When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. Because foods that are boiled are. More energy, as in higher heat, makes molecules move even faster. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature.. Does It Take Longer To Boil Water In High Altitude.
From www.thespruceeats.com
Making Candy at High Altitudes Does It Take Longer To Boil Water In High Altitude It depends on where you’re doing the. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. As the altitude increases, the atmospheric pressure. It seems like one of those basic science facts: Water boils at 212 degrees fahrenheit (100 degrees celsius), right? When atmospheric pressure is lower, such. Does It Take Longer To Boil Water In High Altitude.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does It Take Longer To Boil Water In High Altitude More energy, as in higher heat, makes molecules move even faster. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? Because foods that are boiled are. One of the most significant changes that occur in high altitude areas. Does It Take Longer To Boil Water In High Altitude.
From fyoxouusm.blob.core.windows.net
Does It Take Water Longer To Boil In High Altitude at Joseph May blog Does It Take Longer To Boil Water In High Altitude More energy, as in higher heat, makes molecules move even faster. Less energy means less heat, which means water will boil at a lower. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to. Does It Take Longer To Boil Water In High Altitude.
From www.wonderopolis.org
Why Does Water Boil Faster at Higher Altitude? Wonderopolis Does It Take Longer To Boil Water In High Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. As the altitude increases, the atmospheric pressure. The temperature at which water boils decreases as elevation increases. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower temperature. Less energy means less heat,. Does It Take Longer To Boil Water In High Altitude.
From mugendo.es
How long does it take for water to boil Factors and Implications Does It Take Longer To Boil Water In High Altitude As the altitude increases, the atmospheric pressure. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? It seems like one of those basic science facts: Less energy means less heat, which means water will boil at a lower. It depends on where. Does It Take Longer To Boil Water In High Altitude.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does It Take Longer To Boil Water In High Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Less energy means less heat, which means water will boil at a lower. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? As the altitude increases, the atmospheric pressure. At a higher elevation, the lower atmospheric pressure means heated water reaches its. Does It Take Longer To Boil Water In High Altitude.
From www.wonderopolis.org
Why Does Water Boil Faster at Higher Altitude? Wonderopolis Does It Take Longer To Boil Water In High Altitude The temperature at which water boils decreases as elevation increases. When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? Because foods that are boiled are. Less energy means less heat, which means water will boil at a. Does It Take Longer To Boil Water In High Altitude.
From moralstation.com
How long does it take for water to boil? Know all details related to Does It Take Longer To Boil Water In High Altitude The temperature at which water boils decreases as elevation increases. As the altitude increases, the atmospheric pressure. When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. Because foods that are boiled are. Water boils at 212 degrees fahrenheit (100 degrees celsius), right? When atmospheric pressure is lower, such as at a higher. Does It Take Longer To Boil Water In High Altitude.
From www.tastingtable.com
How To Adjust Your Water Boiling Process For High Altitudes Does It Take Longer To Boil Water In High Altitude When you boil water, you're literally speeding up liquid h20 molecules so much that you're breaking their. As the altitude increases, the atmospheric pressure. More energy, as in higher heat, makes molecules move even faster. One of the most significant changes that occur in high altitude areas concerning cooking is the boiling point of water. The temperature at which water. Does It Take Longer To Boil Water In High Altitude.
From slideplayer.com
AIM 2 How does temperature affect the vapor pressure of a liquid Does It Take Longer To Boil Water In High Altitude When atmospheric pressure is lower, such as at a higher altitude, it takes less energy to bring water to the boiling point. More energy, as in higher heat, makes molecules move even faster. As the altitude increases, the atmospheric pressure. At a higher elevation, the lower atmospheric pressure means heated water reaches its boiling point more quickly—i.e., at a lower. Does It Take Longer To Boil Water In High Altitude.