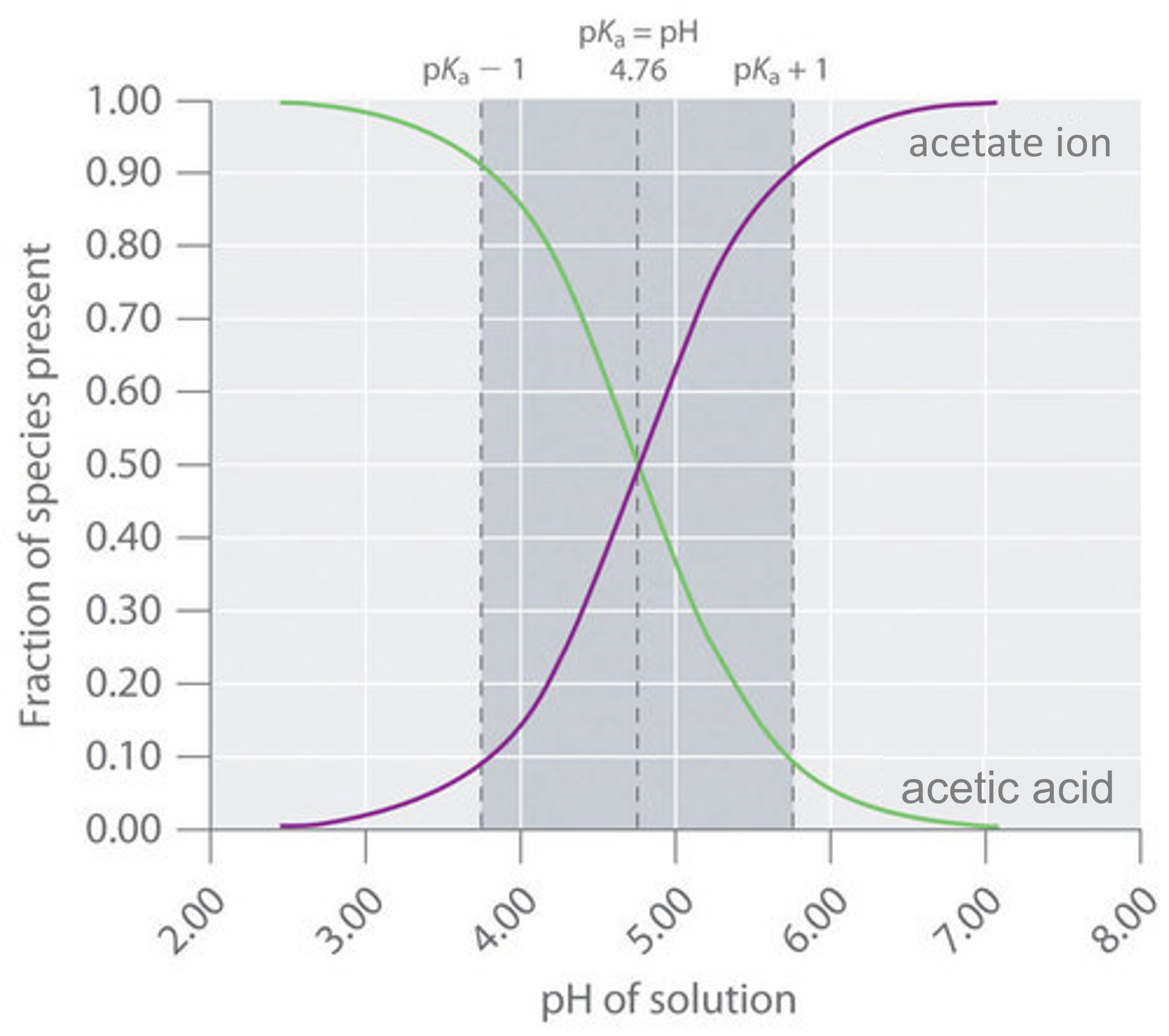
Action Of Acidic Buffer . When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. this page describes simple acidic and alkaline buffer solutions and explains how they work. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer solution is one which. What is a buffer solution? buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. Either a weak acid plus a salt derived from that weak acid, or a weak. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a.
from chem.libretexts.org
this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? Either a weak acid plus a salt derived from that weak acid, or a weak. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A buffer solution is one which. When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a.
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts
Action Of Acidic Buffer this page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which. Either a weak acid plus a salt derived from that weak acid, or a weak. When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. What is a buffer solution? buffers resist dramatic changes in ph by being composed of certain pairs of solutes: buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. this page describes simple acidic and alkaline buffer solutions and explains how they work.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Action Of Acidic Buffer What is a buffer solution? this page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. — a mixture of a weak acid. Action Of Acidic Buffer.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online Action Of Acidic Buffer buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. When a drop of. Action Of Acidic Buffer.
From www.aakash.ac.in
Acidic Buffer Solution Definition, Calculation & Applications Action Of Acidic Buffer A buffer solution is one which. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. What is a buffer solution? When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. buffers resist dramatic changes. Action Of Acidic Buffer.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. — a mixture of a weak acid and. Action Of Acidic Buffer.
From www.youtube.com
buffer actions electrochemistry class 12 chemistry subject notes Action Of Acidic Buffer this page describes simple acidic and alkaline buffer solutions and explains how they work. What is a buffer solution? A buffer solution is one which. Either a weak acid plus a salt derived from that weak acid, or a weak. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: buffer action, in. Action Of Acidic Buffer.
From www.peoi.org
Chapter 12 Section G Buffers Action Of Acidic Buffer buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. this page describes simple acidic and alkaline buffer solutions and explains how they work. A buffer solution is one which. — a mixture of a weak acid and its conjugate base (or a. Action Of Acidic Buffer.
From www.expii.com
Buffers — Definition & Overview Expii Action Of Acidic Buffer buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. A buffer solution is one. Action Of Acidic Buffer.
From brainly.in
What is buffer solution and explain buffer action of acidic buffer Action Of Acidic Buffer Either a weak acid plus a salt derived from that weak acid, or a weak. A buffer solution is one which. this page describes simple acidic and alkaline buffer solutions and explains how they work. When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. buffers resist dramatic changes. Action Of Acidic Buffer.
From www.youtube.com
Buffer Action Acidic and basic buffer Action how buffer resist pH Action Of Acidic Buffer What is a buffer solution? Either a weak acid plus a salt derived from that weak acid, or a weak. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution is one which. this page. Action Of Acidic Buffer.
From exoxulnnf.blob.core.windows.net
Biological Roles Of Buffers at Dana Wild blog Action Of Acidic Buffer When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a.. Action Of Acidic Buffer.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution is one which. Either a weak acid plus a salt derived from that weak acid, or a weak. buffers resist dramatic changes in ph by. Action Of Acidic Buffer.
From www.slideserve.com
PPT Buffer This PowerPoint Presentation, free download ID2841454 Action Of Acidic Buffer When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. this page describes simple acidic and alkaline buffer solutions and explains how they work. What. Action Of Acidic Buffer.
From www.youtube.com
1 Buffers Acidic Buffers YouTube Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. buffer action, in general, is defined as the ability. Action Of Acidic Buffer.
From www.brainkart.com
Buffers Action Of Acidic Buffer A buffer solution is one which. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: What is a buffer solution? When a drop of hcl is added, the. Action Of Acidic Buffer.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffer action,. Action Of Acidic Buffer.
From www.youtube.com
Buffer action in the blood YouTube Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. What is a buffer solution? buffer action, in general,. Action Of Acidic Buffer.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Action Of Acidic Buffer Either a weak acid plus a salt derived from that weak acid, or a weak. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. this page describes simple acidic and alkaline buffer solutions and explains how they. Action Of Acidic Buffer.
From chemistrypubs.com
Buffer Solution Definition, Examples, Mechanisms, Preparations Action Of Acidic Buffer buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. A buffer solution is one which. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a. Action Of Acidic Buffer.
From www.youtube.com
Acidic Buffers Buffer Solution Buffer Action how buffer resist pH Action Of Acidic Buffer buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. Either a weak acid plus a salt derived from that weak acid, or a weak. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and. Action Of Acidic Buffer.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Action Of Acidic Buffer Either a weak acid plus a salt derived from that weak acid, or a weak. What is a buffer solution? this page describes simple acidic and alkaline buffer solutions and explains how they work. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called. Action Of Acidic Buffer.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. What is a buffer solution? buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small. Action Of Acidic Buffer.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Action Of Acidic Buffer Either a weak acid plus a salt derived from that weak acid, or a weak. When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a.. Action Of Acidic Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Action Of Acidic Buffer When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. this page describes simple acidic and alkaline buffer solutions and explains how they work. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. A. Action Of Acidic Buffer.
From users.highland.edu
Buffers Action Of Acidic Buffer A buffer solution is one which. When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. Either a weak acid plus a salt derived from that. Action Of Acidic Buffer.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. What is a buffer solution? buffers resist dramatic changes in ph by being composed of certain pairs of solutes: buffer action, in general, is defined as the ability of the buffer solution. Action Of Acidic Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Action Of Acidic Buffer buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. A buffer solution is one which. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: — a mixture of a weak acid and its conjugate base (or a. Action Of Acidic Buffer.
From www.brainkart.com
Buffers Examples, Buffer action, Key Concept Action Of Acidic Buffer What is a buffer solution? — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution is one which. When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water. Action Of Acidic Buffer.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Action Of Acidic Buffer When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. What is a buffer solution? — a mixture of a weak acid and its conjugate. Action Of Acidic Buffer.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer solution is one which. Either a weak acid plus a salt derived from that weak acid, or a weak. What is a buffer solution? — a. Action Of Acidic Buffer.
From www.youtube.com
Mechanism of Acidic Buffer and Basic Buffer Solution Chemical Action Of Acidic Buffer What is a buffer solution? When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules. A buffer solution is one which. this page describes simple acidic and alkaline buffer solutions and explains how they work. buffers resist dramatic changes in ph by being composed of certain pairs of solutes:. Action Of Acidic Buffer.
From chem.libretexts.org
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts Action Of Acidic Buffer Either a weak acid plus a salt derived from that weak acid, or a weak. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: this page describes simple. Action Of Acidic Buffer.
From www.youtube.com
Acidic Buffers, Basic Buffers, and the Henderson Hasselbalch Equation Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: When a drop of hcl is added, the added h+ ions combine with nh4oh to form undissociated water molecules.. Action Of Acidic Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Action Of Acidic Buffer — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. A buffer solution is. Action Of Acidic Buffer.
From www.pinterest.com
Acidic Buffer and Basic Buffer & its Mechanism Buffer and Buffer Action Of Acidic Buffer buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. A buffer solution is one which. Either a weak acid plus a salt derived from that weak acid, or a weak. What is a buffer solution? buffers resist dramatic changes in ph by being. Action Of Acidic Buffer.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks Action Of Acidic Buffer buffer action, in general, is defined as the ability of the buffer solution to resist the changes in ph value when a small amount. — a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. buffers resist dramatic changes in ph by being. Action Of Acidic Buffer.