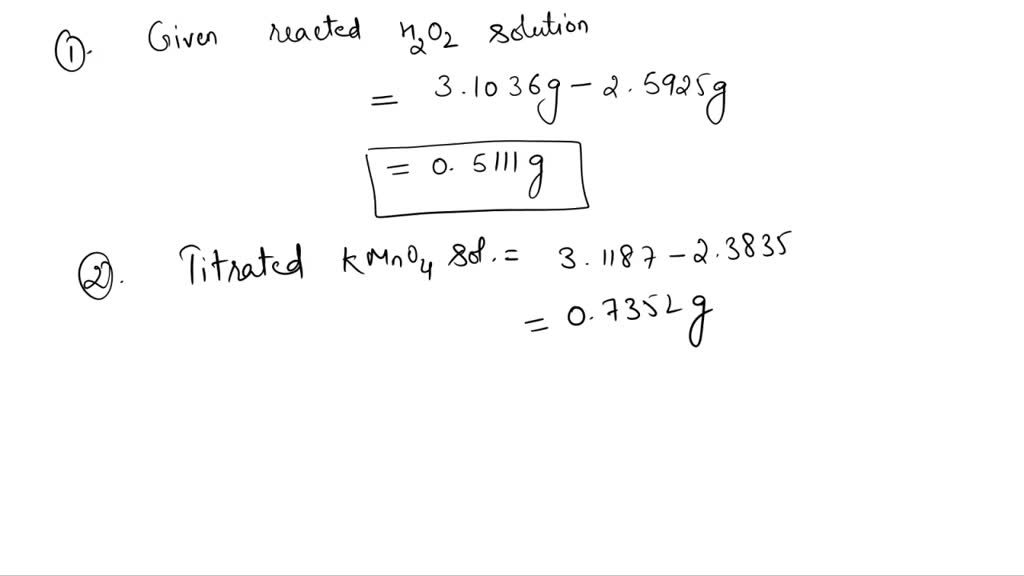Kmno4 Titration Equation . Potassium permanganate is a strong oxidising agent. Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: In an acidic medium, it acts as a powerful oxidising agent. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. Theory of the titration of oxalic acid with kmno4. The oxidising ability of kmno 4 in an acidic. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In close proximity to the endpoint, the action of the. The chemical name for mohr’s salt is ferrous.
from www.numerade.com
Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. Potassium permanganate is a strong oxidising agent. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. In close proximity to the endpoint, the action of the. The oxidising ability of kmno 4 in an acidic. The chemical name for mohr’s salt is ferrous. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution.
SOLVED KMnO4 (Potassium permanganate) = 4.19 (m/m) H2O2 (Hydrogen
Kmno4 Titration Equation Theory of the titration of oxalic acid with kmno4. Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. The chemical name for mohr’s salt is ferrous. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. Potassium permanganate is a strong oxidising agent. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. Theory of the titration of oxalic acid with kmno4. In an acidic medium, it acts as a powerful oxidising agent. The oxidising ability of kmno 4 in an acidic. Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the endpoint, the action of the.
From www.youtube.com
Determination of Concentration of KMnO4 Soution using Ferrous Ammonium Kmno4 Titration Equation In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. Potassium permanganate is a strong oxidising agent. In close proximity to the endpoint, the action of the. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+). Kmno4 Titration Equation.
From www.numerade.com
SOLVED KMnO4 (Potassium permanganate) = 4.19 (m/m) H2O2 (Hydrogen Kmno4 Titration Equation Potassium permanganate is a strong oxidising agent. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In an acidic medium, it acts as a powerful oxidising agent. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h. Kmno4 Titration Equation.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt Kmno4 Titration Equation 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. Potassium permanganate is a strong oxidising agent. The oxidising ability of kmno 4 in an acidic. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration.. Kmno4 Titration Equation.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation ID431911 Kmno4 Titration Equation Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. In close proximity to the endpoint, the action of the. The chemical name for mohr’s salt is ferrous. In an acidic medium, it acts as a powerful oxidising agent. Theory of the titration of oxalic acid with kmno4.. Kmno4 Titration Equation.
From www.coursehero.com
[Solved] A potassium permanganate solution with an approximate molarity Kmno4 Titration Equation Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: The chemical name for mohr’s salt is ferrous. Potassium permanganate is a strong oxidising agent. The titration of potassium permanganate (kmno 4) against. Kmno4 Titration Equation.
From www.numerade.com
SOLVED Potassium permanganate, KMnO4, is a powerful oxidizing agent Kmno4 Titration Equation In close proximity to the endpoint, the action of the. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. Potassium permanganate is a strong oxidising agent. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe. Kmno4 Titration Equation.
From www.numerade.com
SOLVED Write redox halfequations for the reduction MnO4(aq) to Mn2 Kmno4 Titration Equation In an acidic medium, it acts as a powerful oxidising agent. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. The oxidising ability of kmno 4 in an acidic. Theory of the titration of oxalic acid with kmno4. In this experiment you will use a standard solution. Kmno4 Titration Equation.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Kmno4 Titration Equation The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the endpoint, the action of the. Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. The chemical name for mohr’s salt. Kmno4 Titration Equation.
From www.slideserve.com
PPT Variable oxidation states PowerPoint Presentation, free download Kmno4 Titration Equation Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. In an acidic medium, it acts as a powerful oxidising agent. Potassium permanganate is a strong oxidising agent. Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic. Kmno4 Titration Equation.
From www.youtube.com
titration of kmno4 with mohr's salt class 12 determine the Kmno4 Titration Equation In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: Potassium permanganate. Kmno4 Titration Equation.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Kmno4 Titration Equation The chemical name for mohr’s salt is ferrous. Theory of the titration of oxalic acid with kmno4. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o.. Kmno4 Titration Equation.
From fphoto.photoshelter.com
science chemistry titration potassium permanganate Fundamental Kmno4 Titration Equation The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the endpoint, the action of the. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. Theory of the titration of oxalic. Kmno4 Titration Equation.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with Kmno4 Titration Equation 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the. Kmno4 Titration Equation.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Kmno4 Titration Equation In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. In an acidic medium, it acts as a powerful oxidising agent. The. Kmno4 Titration Equation.
From www.youtube.com
How to Write the Net Ionic Equation for H2O2 + KMnO4 + H2SO4 YouTube Kmno4 Titration Equation In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Potassium permanganate is a strong oxidising agent. Potassium permanganate (kmno 4) is. Kmno4 Titration Equation.
From www.chegg.com
Solved 4. In this experiment; as you perform redox titration Kmno4 Titration Equation The chemical name for mohr’s salt is ferrous. Potassium permanganate is a strong oxidising agent. Theory of the titration of oxalic acid with kmno4. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In this titration process, the oxalic acid acts as the. Kmno4 Titration Equation.
From www.chegg.com
Solved Nazl204 KMnO4 Ex. 2 Titration of 0.2121 g of pure Kmno4 Titration Equation In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. Potassium permanganate is a strong oxidising agent. Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be. Kmno4 Titration Equation.
From www.numerade.com
SOLVED Consider different titrations for this exercise. Potassium Kmno4 Titration Equation Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: In close proximity to the endpoint, the action of the. The chemical name for mohr’s salt is ferrous. In this experiment you will. Kmno4 Titration Equation.
From www.tessshebaylo.com
Hydrogen Peroxide Chemical Equation Tessshebaylo Kmno4 Titration Equation The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent. Kmno4 Titration Equation.
From www.numerade.com
SOLVED 0.04M of KMnO4 solution was titrated with 1.00 ml of (13.4 g Kmno4 Titration Equation Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in. Kmno4 Titration Equation.
From www.congress-intercultural.eu
To Determine The Molarity Of KMnO4 Solution By Titrating It, 44 OFF Kmno4 Titration Equation Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. Potassium permanganate is a strong oxidising agent. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2. Kmno4 Titration Equation.
From www.youtube.com
Calculation of KMnO4 and Oxalic acid titration YouTube Kmno4 Titration Equation The oxidising ability of kmno 4 in an acidic. Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so. Kmno4 Titration Equation.
From www.youtube.com
Ammonium Iron (II) Sulphate vs KMnO4 Titration Calculations Example Kmno4 Titration Equation Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. Theory. Kmno4 Titration Equation.
From www.youtube.com
General Chemistry Standardization of KMnO4 By Redox Titration YouTube Kmno4 Titration Equation In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The oxidising ability of kmno 4 in an acidic. Potassium permanganate (kmno 4). Kmno4 Titration Equation.
From www.numerade.com
SOLVED Potassium permanganate reacts with sodium oxalate by the Kmno4 Titration Equation In close proximity to the endpoint, the action of the. The chemical name for mohr’s salt is ferrous. The oxidising ability of kmno 4 in an acidic. Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. The titration of potassium permanganate (kmno 4) against oxalic acid (c. Kmno4 Titration Equation.
From tuitiontube.com
Balancing CaC2O4 + H2SO4 + KMnO4 = K2SO4 + CaSO4 + MnSO4 + H2O + CO2 Kmno4 Titration Equation In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The chemical name for mohr’s salt is ferrous. In close proximity to the endpoint, the action of the. Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2. Kmno4 Titration Equation.
From mavink.com
H Kmno4 Kmno4 Titration Equation In an acidic medium, it acts as a powerful oxidising agent. Potassium permanganate is a strong oxidising agent. In close proximity to the endpoint, the action of the. Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. The titration of potassium permanganate (kmno 4) against oxalic acid. Kmno4 Titration Equation.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Kmno4 Titration Equation Theory of the titration of oxalic acid with kmno4. Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. The oxidising ability of kmno 4 in. Kmno4 Titration Equation.
From slideplayer.com
Balance the following MnO4 Mn2+ Fe2+ Fe ppt download Kmno4 Titration Equation Theory of the titration of oxalic acid with kmno4. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. Potassium permanganate (kmno 4). Kmno4 Titration Equation.
From studylib.net
Experiment 8 Redox Titrations Potassium permanganate, KMnO4 Kmno4 Titration Equation The oxidising ability of kmno 4 in an acidic. In an acidic medium, it acts as a powerful oxidising agent. Theory of the titration of oxalic acid with kmno4. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Mohr salt is a double. Kmno4 Titration Equation.
From www.youtube.com
Titration KMnO4 Vs Mohr Salt in Hindi Full Experiment Kmno4 Titration Equation In an acidic medium, it acts as a powerful oxidising agent. Theory of the titration of oxalic acid with kmno4. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4. Kmno4 Titration Equation.
From brunofuga.adv.br
What Happens When KMnO4 Reacts With FeSO4? Will KMnO4 Get, 46 OFF Kmno4 Titration Equation The oxidising ability of kmno 4 in an acidic. Mohr salt is a double salt forming a single crystalline structure having the formula feso 4.(nh 4) 2 so 4.6h 2 o. The chemical name for mohr’s salt is ferrous. In close proximity to the endpoint, the action of the. Potassium permanganate is a strong oxidising agent. In an acidic medium,. Kmno4 Titration Equation.
From www.youtube.com
Chemistry Redox Reaction Iron (II) (Fe2+) ion with permanganate ion Kmno4 Titration Equation In close proximity to the endpoint, the action of the. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. Theory of the titration of oxalic acid with kmno4. In an acidic medium, it acts as a powerful oxidising agent. The titration of potassium permanganate (kmno 4) against. Kmno4 Titration Equation.
From www.studypool.com
SOLUTION Experiment prepare m 50 solution of oxalic acid with its help Kmno4 Titration Equation The chemical name for mohr’s salt is ferrous. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. 2kmno 4 + 3h 2 so 4 + 5(cooh) 2 → k 2 so 4 + 2mnso 4 + 8h 2 o. In an acidic medium, it acts as a. Kmno4 Titration Equation.
From mungfali.com
Acid Base Titration Formula Kmno4 Titration Equation Theory of the titration of oxalic acid with kmno4. The oxidising ability of kmno 4 in an acidic. Potassium permanganate (kmno 4) is a strong oxidizing agent and it acts as a powerful oxidizing agent in an acidic medium during the titration of oxalic acid with kmno4 that can be represented by the following equation: In close proximity to the. Kmno4 Titration Equation.