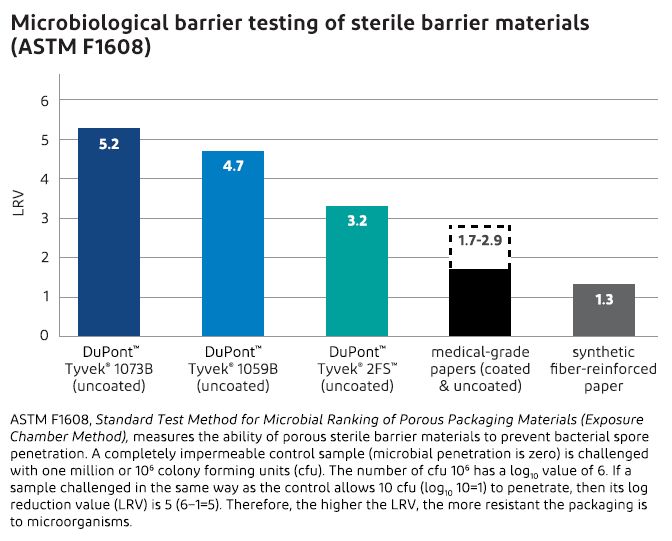
Microbial Barrier Testing Method . The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging.
from www.dupont.co.jp
The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation.
Microbial Barrier DuPont™ Tyvek® for Medical Packaging DuPont USA
Microbial Barrier Testing Method Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation.
From microbiologyguideritesh.com
Flow Chart of Microbial Limit Test Microbiology Guidelines Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Microbial Barrier Testing Method.
From www.integratedpharmaservices.com
Microbial Barrier Testing of Novel Composite Dressings Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From www.pp.dupont.com
Microbial Barrier DuPont™ Tyvek® for Medical Packaging Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From www.researchgate.net
Microbial, physical, chemical, and innate and adaptive immune barrier Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From www.slideserve.com
PPT Innate Immune Response PowerPoint Presentation, free download Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From nationaleczema.org
Skin Barrier Basics for People With Eczema National Eczema Association Microbial Barrier Testing Method Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From www.researchgate.net
(PDF) Determination of the Efficacy of Sterile Barrier Systems Against Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From mycoscience.com
MycoScience Microbial Aerosol Challenge Testing vs. Microbial Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From gardening.gov.capital
How do microbial barriers control pests? Gardening.Gov.Capital Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From www.slideserve.com
PPT Breaking the Rapid Microbiological Method Financial Barrier A Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From www2.mdpi.com
Polymers Free FullText Application of 3DWoven Fabrics for Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From gardening.gov.capital
What are microbial barriers? Gardening.Gov.Capital Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Microbial Barrier Testing Method.
From microbeonline.com
Bacteriological Analysis of Water • Microbe Online Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From www.researchgate.net
(PDF) Liquid Immersion Microbial Challenge Tests Microbial Testing for Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From www.criver.com
Breaking Barriers How Next Generation Sequencing Is Transforming Microbial Barrier Testing Method Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From microbe-investigations.com
USP 61 Testing for Microbial Enumeration Microbe Investigations Microbial Barrier Testing Method Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Microbial Barrier Testing Method.
From eureka.patsnap.com
Dry barrier tester and dry barrier testing Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Microbial Barrier Testing Method.
From www.youtube.com
Superior Microbial Barrier Protection Tyvek® Medical & Pharmaceutical Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From www.researchgate.net
Four components of the intestinal barrier. The microbiological barrier Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From dokumen.tips
(PDF) Microbial Barrier Testing ASTM International Standards Microbial Barrier Testing Method Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From mandflab.com
Microbial Testing Service Medical and Food Lab Co., Ltd. Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From www.slideserve.com
PPT Permeable Reactive Barriers PowerPoint Presentation, free Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From va-kompetanse.no
A 202 Microbial barrier analysis (MBA)—a guideline Norsk Vanns Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Microbial Barrier Testing Method.
From www.researchgate.net
Schematic representation of testing microbial barrier permeability Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From pacificbiolabs.com
Microbial Limits Test Pacific BioLabs Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From slideplayer.com
The innate immune response ppt download Microbial Barrier Testing Method Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From www.dupont.co.jp
Microbial Barrier DuPont™ Tyvek® for Medical Packaging DuPont USA Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From silab.fr
Immunity, essential ally of skin beauty SILAB Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From jnjinstitute.com
Watertight, Microbial Barrier Protection for Your Patients Johnson Microbial Barrier Testing Method Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From www.youtube.com
Role of Microbial Barriers In Infection Prevention YouTube Microbial Barrier Testing Method Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From www.researchgate.net
Schematic of microbial barrier permeability testing. Download Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Microbial Barrier Testing Method.
From www.cmghjournal.org
Regulation of Intestinal Barrier Function by Microbial Metabolites Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Defining the range of microbiological barrier performances of commercially available porous packaging. Microbial Barrier Testing Method.
From sterilebarrier.org
Managing Risks Sterile Barrier Microbial Barrier Testing Method The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Defining the range of microbiological barrier performances of commercially available porous packaging. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From www.rapidmicrobio.com
barriers to adoption of rapid microbial testing Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.
From www.science.org
Microbiota and maintenance of skin barrier function Science Microbial Barrier Testing Method Defining the range of microbiological barrier performances of commercially available porous packaging. The main value of the astm f1608 test method lies in its ability to provide quantitative data on the material's microbial barrier properties. Research work on microbial barrier performance of porous medical packaging materials started in 1998 and led to the creation. Microbial Barrier Testing Method.