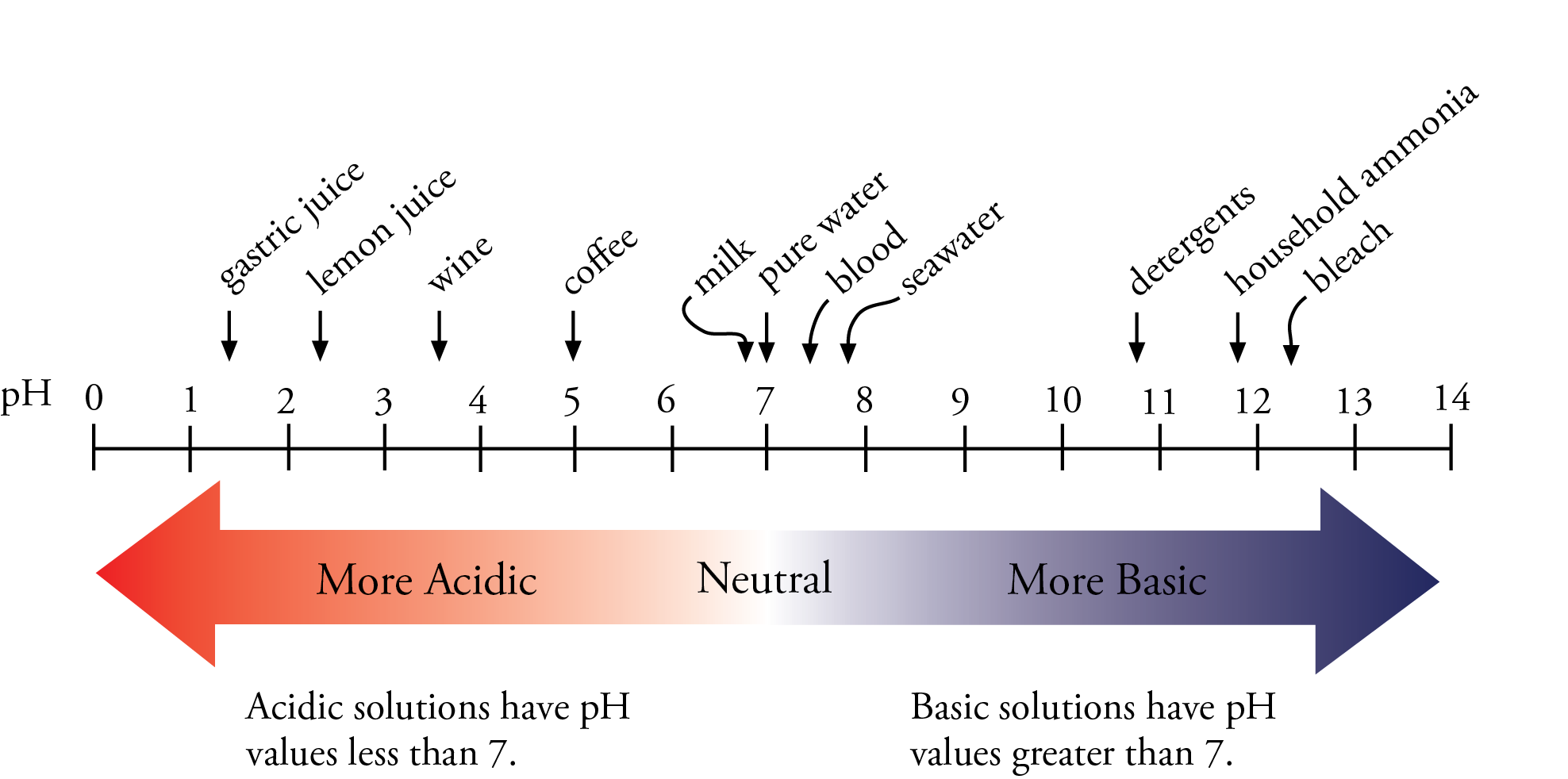
The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution . To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. Ph & poh of strong acids & bases strong acids. The equation for ph is: Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The letters ph stand for the power of the hydrogen ion.
from preparatorychemistry.com
The letters ph stand for the power of the hydrogen ion. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The equation for ph is: Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. Ph & poh of strong acids & bases strong acids.
pH and Equilibrium
The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The letters ph stand for the power of the hydrogen ion. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The letters ph stand for the power of the hydrogen ion. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Ph & poh of strong acids & bases strong acids. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The equation for ph is:
From acidsandbasesrios.weebly.com
pH Acids and Bases The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. The letters ph stand for the power of the hydrogen ion. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The equation for ph is: Ph & poh of strong acids &. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From ournutritionkitchen.com
Think fast what the pH? The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Ph & poh of strong acids & bases strong acids. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From quizdbcornwallis.z21.web.core.windows.net
What Type Of Ions Do Acids Release The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Ph & poh of strong acids & bases strong acids. The equation for ph is: The letters ph stand for the power of the hydrogen ion. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. Today, we know that acids produce hydronium ions in solution rather. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The equation for ph is: Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. Ph & poh of strong acids & bases. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From sciencenotes.org
The pH Scale of Common Chemicals The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The equation for ph is: Ph & poh of strong acids & bases strong acids. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. The letters ph stand for the power of the hydrogen ion. Most strong acids are monoprotic and fully ionize in aqueous solutions. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.oist.jp
pH Values of Common Substances Okinawa Institute of Science and The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Ph & poh of strong acids & bases strong acids. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The letters ph stand for the power of the hydrogen ion. To determine the strength of acids and bases. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From study.com
Acidic, Basic & Neutral Solutions Determining pH Video & Lesson The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. The equation for ph is: The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Ph & poh of strong acids & bases strong acids. The letters ph stand for. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.shalom-education.com
Acids and Alkalis GCSE Chemistry Revision The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The equation for ph is: Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. Ph & poh of strong acids & bases. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From socratic.org
The pH of a 2M solution of NaOH is...? (please include the complete The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The letters ph stand for the power of the hydrogen ion. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The equation for ph is:. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. The letters ph stand for the power. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.nagwa.com
Question Video Calculating the pH of a Solution with a Given Hydronium The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Ph & poh of strong acids & bases strong acids. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The letters ph. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From magickutta.blogspot.com
magickudam ACID and ALKALIS The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The letters ph stand for the power of the hydrogen ion. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. Ph & poh of strong acids & bases strong acids. The numbers of the ph scale come from a. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From ar.inspiredpencil.com
Ph Examples Of Acids And Bases The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The letters ph stand for the power of the hydrogen ion. The equation for ph is: The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. Ph & poh of strong acids & bases strong acids. Today, we know. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.youtube.com
CHEMISTRY 201 Calculating the pH of a weak acid solution YouTube The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The letters ph stand for the power of the hydrogen ion. Ph &. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From preparatorychemistry.com
pH and Equilibrium The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The equation for ph is: Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The letters ph stand for the power of the hydrogen ion. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. Today, we know that acids produce hydronium. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From socratic.org
What is the pH of a .001 M solution of HCl? Socratic The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The letters ph stand for the power of the hydrogen ion. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. To determine the strength of. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.vecteezy.com
The chart shows the Acidic Neutral and Alkaline pH of various liquids The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Ph & poh of strong acids & bases strong acids. The equation for ph is: To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.ck12.org
Acids and Bases CK12 Foundation The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. The equation for ph is: Ph & poh of strong acids & bases strong acids. The letters ph stand for. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From courses.lumenlearning.com
Compounds Essential to Human Functioning Anatomy and The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. The letters ph stand for the power of the hydrogen ion. The equation for ph is: Most strong acids are. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.alamy.com
ph scale. chart of pH value for acid and alkaline solutions. acidbase The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The letters ph stand for the power of the hydrogen ion. Ph & poh of strong acids & bases strong acids. The equation for ph is: To determine the. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.askiitians.com
Amino Acids Properties Study Material for IIT JEE askIITians The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. The letters ph stand for the. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The equation for ph is: Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The numbers of the ph scale come from a formula for. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Ph & poh of strong acids & bases strong acids. The equation for ph is: The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. To determine the strength of acids and bases quantitatively, we use a universal indicator. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.pmel.noaa.gov
The pH scale by numbers The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The equation for ph is: The letters ph stand for the power of the hydrogen ion. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The numbers of the ph scale come from a formula for the hydrogen ion. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From mungfali.com
Hydrogen Ions And PH The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. Ph & poh of strong acids &. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.dreamstime.com
Scale of Ph Value for Acid and Alkaline Solutions Stock Vector The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The equation for ph is: Ph &. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.numerade.com
SOLVED In chemistry, the pH scale measures the acidity of a solution The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The equation for ph is: Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. Ph & poh of strong acids & bases strong acids. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.expii.com
What Is pH? — Definition & Overview Expii The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The equation for ph is: To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The letters ph stand for the power of the hydrogen ion. Ph & poh of strong acids &. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.alamy.com
Acidic And Alkaline Solution pH Value Scale Chart. Infographic Balance The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The letters ph stand for the power of the hydrogen ion. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at different concentrations of hydrogen. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Ph & poh of strong acids & bases strong. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From in.pinterest.com
pH chart Skins ph, Acid base, Carbonated drinks The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Ph & poh of strong acids & bases strong acids. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include hcl. The letters ph stand for the. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.epa.gov
Understanding the Science of Ocean and Coastal Acidification Ocean The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. Ph & poh of strong acids & bases strong acids. The letters ph. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From pediaa.com
Relationship Between Hydrogen Ions and pH Definition, Properties, pH The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. Most strong acids are monoprotic and fully ionize in aqueous solutions examples include. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.careerpower.in
pH Values List for Common Substances The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The letters ph stand for the power of the hydrogen ion. The equation for ph is: The numbers of the ph scale come from a formula for the hydrogen ion. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.bbc.co.uk
What is the pH scale and what does it measure? BBC Bitesize The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The letters ph stand for the power of the hydrogen ion. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colours at. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.
From www.alamy.com
vector ph scale of acidic,neutral and alkaline value chart for acid and The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution Today, we know that acids produce hydronium ions in solution rather than hydrogen ions, so the definition of ph may be slightly reworded to be the negative logarithm of the. The numbers of the ph scale come from a formula for the hydrogen ion (h +) concentration. The letters ph stand for the power of the hydrogen ion. Most strong. The Ph Value Of An Acid Represents Its Of Positive Ions In The Solution.