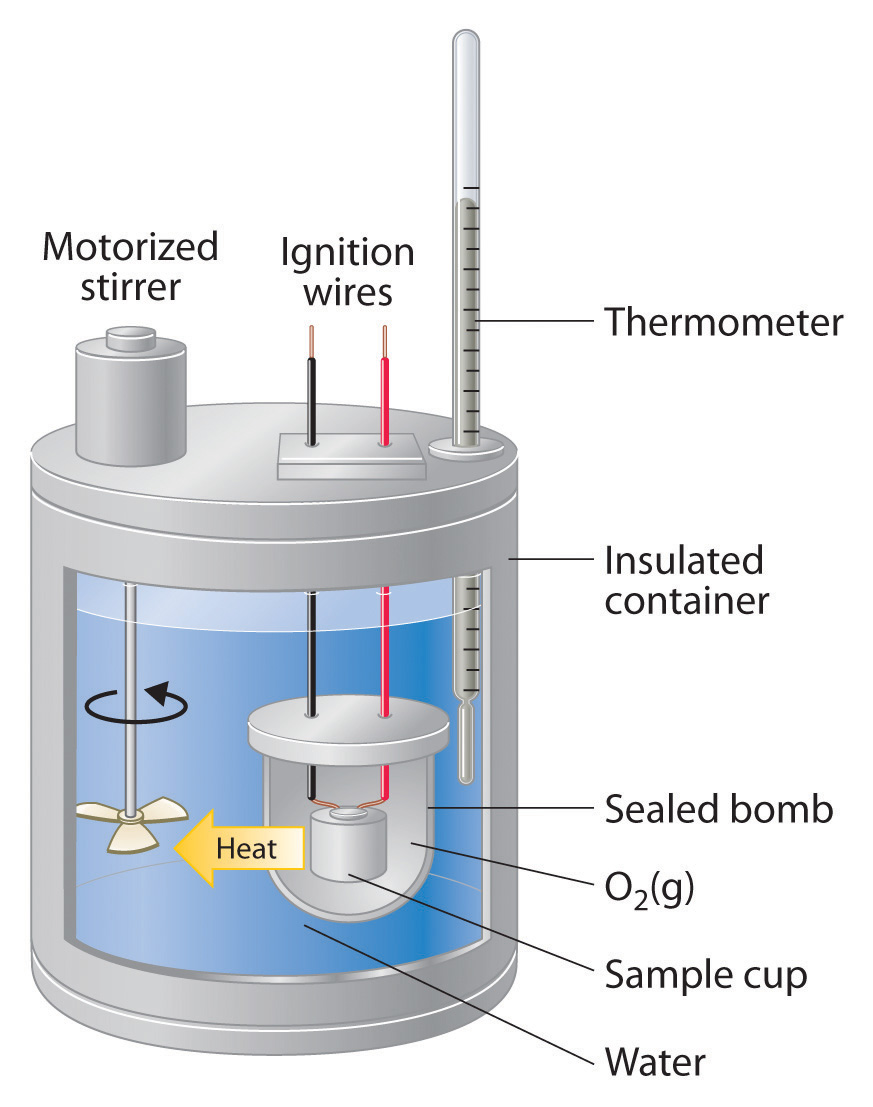
What Are The Possible Sources Of Error In A Calorimetry Experiment . Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Suppose we initially have a high. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. There are many potential sources of error in calorimetry experiments, both quantitative and qualitative. More reliable results can be obtained by repeating the experiment many times. Not all the heat produced by the combustion reaction is transferred to the water. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Some heat is lost to the surroundings; Suppose we initially have a high. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry.
from sites.google.com
Some heat is lost to the surroundings; Suppose we initially have a high. More reliable results can be obtained by repeating the experiment many times. Suppose we initially have a high. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. There are many potential sources of error in calorimetry experiments, both quantitative and qualitative. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Not all the heat produced by the combustion reaction is transferred to the water.
Calorimetry Preliminary HSC Chemistry
What Are The Possible Sources Of Error In A Calorimetry Experiment The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Not all the heat produced by the combustion reaction is transferred to the water. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. More reliable results can be obtained by repeating the experiment many times. Suppose we initially have a high. Some heat is lost to the surroundings; Suppose we initially have a high. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. There are many potential sources of error in calorimetry experiments, both quantitative and qualitative. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating.
From chemistnotes.com
Errors in Chemical Analysis Determinate and Indeterminate Errors What Are The Possible Sources Of Error In A Calorimetry Experiment Not all the heat produced by the combustion reaction is transferred to the water. Some heat is lost to the surroundings; More reliable results can be obtained by repeating the experiment many times. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Suppose we initially have a high.. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.numerade.com
SOLVED Name three possible sources of error in a calorimetry experiment. What Are The Possible Sources Of Error In A Calorimetry Experiment The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Suppose we initially have a high. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.youtube.com
Effects of experimental errors on enthalpy measurements YouTube What Are The Possible Sources Of Error In A Calorimetry Experiment Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Not all the heat produced by the combustion reaction is transferred to the water. More reliable results can be obtained. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.slideserve.com
PPT Heat of Combustion PowerPoint Presentation ID6750144 What Are The Possible Sources Of Error In A Calorimetry Experiment Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Some heat is lost to the surroundings; More reliable results can be obtained by repeating the experiment many times. Not all the heat produced by the combustion reaction is transferred to the water. There are many potential sources of. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.numerade.com
SOLVED Enthalpy of Neutralization Results Sheet Note The procedure is What Are The Possible Sources Of Error In A Calorimetry Experiment Not all the heat produced by the combustion reaction is transferred to the water. There are many potential sources of error in calorimetry experiments, both quantitative and qualitative. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. More reliable results can be obtained by repeating the. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.biologyforlife.com
Error Analysis BIOLOGY FOR LIFE What Are The Possible Sources Of Error In A Calorimetry Experiment Suppose we initially have a high. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Suppose we initially have a high. There are many potential sources of error in calorimetry experiments, both quantitative and qualitative. More reliable results can be obtained by repeating the experiment many times. Learn why all. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.baamboozle.com
Heat Gain and Heat Loss Baamboozle Baamboozle The Most Fun What Are The Possible Sources Of Error In A Calorimetry Experiment Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Not all the heat produced by the combustion reaction is transferred to the water. Suppose we initially have a high.. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube What Are The Possible Sources Of Error In A Calorimetry Experiment Some heat is lost to the surroundings; More reliable results can be obtained by repeating the experiment many times. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. The student used 25.372 g for this. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.slideserve.com
PPT Errors and Uncertainties PowerPoint Presentation, free download What Are The Possible Sources Of Error In A Calorimetry Experiment Suppose we initially have a high. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Not all the heat produced by the combustion reaction is transferred to the water. Suppose we initially have a high. There are many potential sources of error in calorimetry experiments, both quantitative and. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson What Are The Possible Sources Of Error In A Calorimetry Experiment Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Suppose we initially have a high. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Not all the heat produced by the combustion reaction is transferred to the water.. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From philschatz.com
Calorimetry · Chemistry What Are The Possible Sources Of Error In A Calorimetry Experiment Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. There are many potential sources of error in calorimetry experiments, both quantitative and qualitative. Some heat. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From chemistrytalk.org
Calorimetry ChemTalk What Are The Possible Sources Of Error In A Calorimetry Experiment More reliable results can be obtained by repeating the experiment many times. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Suppose we initially have a high. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. There are many potential. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment What Are The Possible Sources Of Error In A Calorimetry Experiment Not all the heat produced by the combustion reaction is transferred to the water. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Suppose we initially have a high. Some heat is lost to the. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.slideshare.net
4 Calorimetry What Are The Possible Sources Of Error In A Calorimetry Experiment Suppose we initially have a high. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Not all the heat produced by the combustion reaction is transferred to the water. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.slideshare.net
4 Calorimetry What Are The Possible Sources Of Error In A Calorimetry Experiment There are many potential sources of error in calorimetry experiments, both quantitative and qualitative. Some heat is lost to the surroundings; Suppose we initially have a high. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Not all the heat produced by the combustion reaction is. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.chegg.com
Solved EXPERIMENT 8 ab Questions post osible sources of What Are The Possible Sources Of Error In A Calorimetry Experiment Suppose we initially have a high. Some heat is lost to the surroundings; Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Not all the heat produced. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.slideserve.com
PPT Chapter 14 Heat PowerPoint Presentation, free download ID6832402 What Are The Possible Sources Of Error In A Calorimetry Experiment The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Not all the heat produced by the combustion reaction is transferred to the water. Suppose we initially have a high. Some heat is lost to the surroundings; Before we practice calorimetry problems involving chemical reactions, consider a. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.numerade.com
Name three possible sources of error in a calorimetry experiment What Are The Possible Sources Of Error In A Calorimetry Experiment Some heat is lost to the surroundings; The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Not all the heat produced by the combustion reaction is transferred. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From syatillakmk.blogspot.com
SimplyChemistry Sources of errors in titration What Are The Possible Sources Of Error In A Calorimetry Experiment The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Suppose we initially have a high. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From grade12uchem.weebly.com
Calorimetry latest copy of grade 12 U What Are The Possible Sources Of Error In A Calorimetry Experiment The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Suppose. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From sites.google.com
3.2.1 (e) Q=mcΔT Ellesmere OCR A level Chemistry What Are The Possible Sources Of Error In A Calorimetry Experiment Suppose we initially have a high. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Some heat is lost to the surroundings; Suppose we initially have a high. Before we practice. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube What Are The Possible Sources Of Error In A Calorimetry Experiment Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. More reliable results can be obtained by repeating the experiment many times. The biggest source of error in calorimetry is usually unwanted. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From brainly.com
What possible source of errors would be in this experiment besides What Are The Possible Sources Of Error In A Calorimetry Experiment Not all the heat produced by the combustion reaction is transferred to the water. More reliable results can be obtained by repeating the experiment many times. Some heat is lost to the surroundings; The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Suppose we initially have a high. Suppose we initially have a high.. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.nagwa.com
Question Video Identifying the Factor That Does Not Need to Be Held What Are The Possible Sources Of Error In A Calorimetry Experiment Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Not all the heat produced by the combustion reaction is transferred to the water. More reliable results can be obtained by repeating the experiment many times. Some heat is lost to the surroundings; The student used 25.372 g for. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.nagwa.com
Question Video Determining the Correct Formula to Use in Order to What Are The Possible Sources Of Error In A Calorimetry Experiment Suppose we initially have a high. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Suppose we initially have a high. Some heat is lost to the surroundings; Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. More. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.researchgate.net
Sources of error in calorimetry measurements Download Scientific Diagram What Are The Possible Sources Of Error In A Calorimetry Experiment The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Some heat is lost to the surroundings; Suppose we initially have a high. The student used 25.372 g for this experiment but recorded 23.372 g in. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.chegg.com
Solved 4) The calorimeter used in this experiment has no What Are The Possible Sources Of Error In A Calorimetry Experiment Some heat is lost to the surroundings; The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. More reliable results can be obtained by repeating the experiment many times. Not all the heat produced by the combustion reaction is transferred to the water. Before we practice calorimetry problems involving chemical reactions, consider a simple example. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.coursehero.com
[Solved] i need help with this The Fundamentals of Calorimetry Data What Are The Possible Sources Of Error In A Calorimetry Experiment Suppose we initially have a high. More reliable results can be obtained by repeating the experiment many times. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. Not all the heat produced by the combustion reaction is transferred to the water. Before we practice calorimetry problems involving chemical. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From ohmlaw.com
5 Error Sources in Ohm's Law Experiment [How to avoid them] • Ohm Law What Are The Possible Sources Of Error In A Calorimetry Experiment The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. There are many potential sources of error in calorimetry experiments, both quantitative and qualitative. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Learn why all science experiments have error, how. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment What Are The Possible Sources Of Error In A Calorimetry Experiment Not all the heat produced by the combustion reaction is transferred to the water. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Suppose we initially have. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment What Are The Possible Sources Of Error In A Calorimetry Experiment Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Not all the heat produced by the combustion reaction is transferred to the water. Some heat. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From sites.google.com
Calorimetry Preliminary HSC Chemistry What Are The Possible Sources Of Error In A Calorimetry Experiment More reliable results can be obtained by repeating the experiment many times. Suppose we initially have a high. The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. The student used 25.372 g for. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.pinterest.co.uk
Pin on Science Friday What Are The Possible Sources Of Error In A Calorimetry Experiment The biggest source of error in calorimetry is usually unwanted heat loss to the surroundings. Suppose we initially have a high. Suppose we initially have a high. Learn why all science experiments have error, how to calculate it, and the sources and types of errors you should report. More reliable results can be obtained by repeating the experiment many times.. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 What Are The Possible Sources Of Error In A Calorimetry Experiment The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Before we practice calorimetry problems involving chemical reactions, consider a simpler example that illustrates the core idea behind calorimetry. Suppose we initially have a high. Learn why all science experiments have error, how to calculate it, and. What Are The Possible Sources Of Error In A Calorimetry Experiment.
From classlistnoon.z13.web.core.windows.net
Coffee Cup Calorimetry Experiment What Are The Possible Sources Of Error In A Calorimetry Experiment Some heat is lost to the surroundings; The student used 25.372 g for this experiment but recorded 23.372 g in the data table and used the incorrect value when calculating. Before we practice calorimetry problems involving chemical reactions, consider a simple example that illustrates the core idea behind calorimetry. Suppose we initially have a high. Suppose we initially have a. What Are The Possible Sources Of Error In A Calorimetry Experiment.