What Happens When Limestone Reacts With Acid . But if you add an acid, you add hydrogen ions (h+), which will react with the carbonate to form In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. Limestone is mostly made up of the mineral calcium carbonate (caco3). What happens when acid is added to limestone? As acid rain falls to the earth's surface, limestone rocks and limestone components in soil will react with the rain, neutralize the acid and dissolve. The calcium chloride produced is very soluble in water, and acid can thus speed. Burning fossil fuels also contributes to this. This causes the limestone to dissolve. This is not very soluble, so rocks don't dissolve very quickly. As the limestone dissolves, the rocks will wear. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone.
from www.nagwa.com
As the limestone dissolves, the rocks will wear. Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. Burning fossil fuels also contributes to this. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. This causes the limestone to dissolve. As acid rain falls to the earth's surface, limestone rocks and limestone components in soil will react with the rain, neutralize the acid and dissolve. Limestone is mostly made up of the mineral calcium carbonate (caco3). In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off.
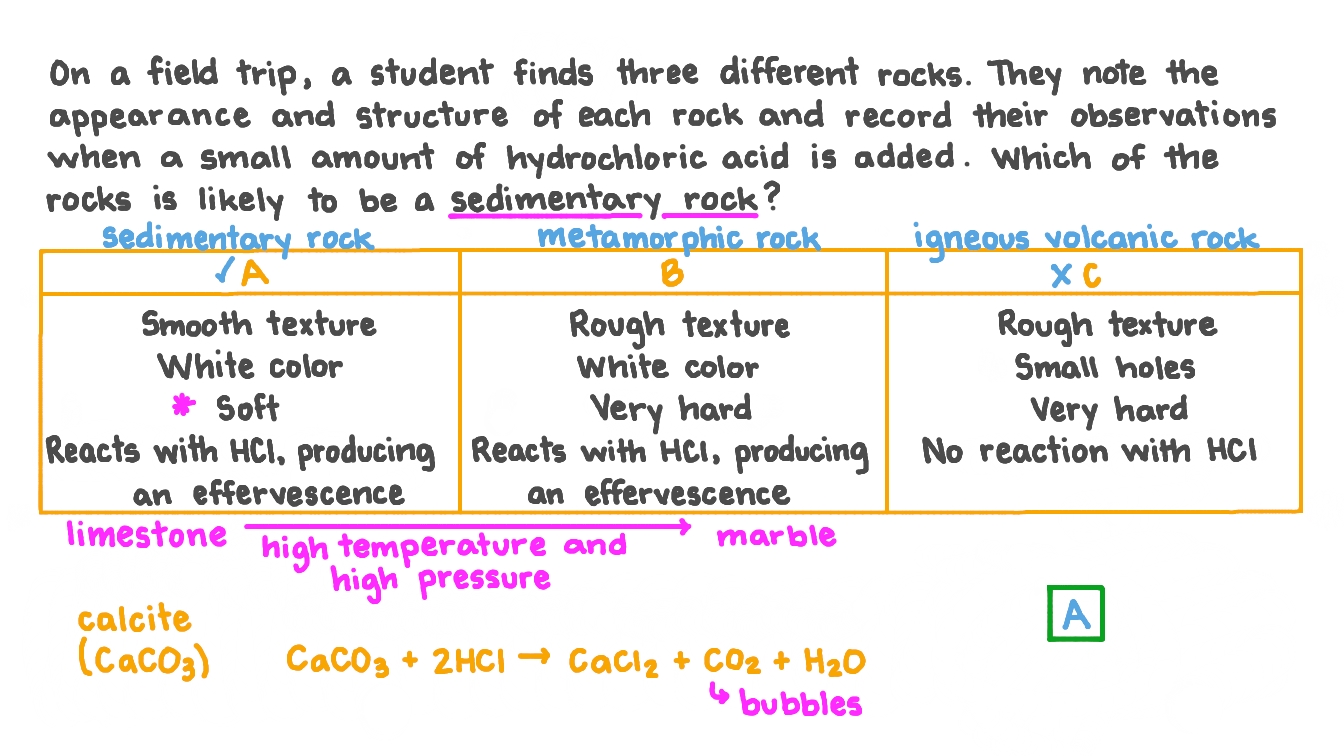
Question Video Identifying a Sedimentary Rock from Its Appearance
What Happens When Limestone Reacts With Acid Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. As the limestone dissolves, the rocks will wear. In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. What happens when acid is added to limestone? When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. As acid rain falls to the earth's surface, limestone rocks and limestone components in soil will react with the rain, neutralize the acid and dissolve. Burning fossil fuels also contributes to this. Limestone is mostly made up of the mineral calcium carbonate (caco3). This causes the limestone to dissolve. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. The calcium chloride produced is very soluble in water, and acid can thus speed. Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. This is not very soluble, so rocks don't dissolve very quickly. But if you add an acid, you add hydrogen ions (h+), which will react with the carbonate to form
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with What Happens When Limestone Reacts With Acid Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. This causes the limestone to dissolve. Burning fossil fuels also contributes to this. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. When acid reacts with limestone, a chemical reaction occurs where the acid. What Happens When Limestone Reacts With Acid.
From cerzztyd.blob.core.windows.net
What Happens When Base React With Carbonate at Jackie Sharpe blog What Happens When Limestone Reacts With Acid Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. As the limestone dissolves, the rocks will wear. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic. What Happens When Limestone Reacts With Acid.
From pixels.com
Limestone Reacting With Acid Photograph by Science Photo Library Pixels What Happens When Limestone Reacts With Acid Burning fossil fuels also contributes to this. But if you add an acid, you add hydrogen ions (h+), which will react with the carbonate to form Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. What happens when acid is added to limestone? As acid rain falls to the earth's. What Happens When Limestone Reacts With Acid.
From diya-jolpblogcooper.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid What Happens When Limestone Reacts With Acid As the limestone dissolves, the rocks will wear. What happens when acid is added to limestone? But if you add an acid, you add hydrogen ions (h+), which will react with the carbonate to form The calcium chloride produced is very soluble in water, and acid can thus speed. As acid rain falls to the earth's surface, limestone rocks and. What Happens When Limestone Reacts With Acid.
From mavink.com
Calcium Reacts With Hydrochloric Acid What Happens When Limestone Reacts With Acid But if you add an acid, you add hydrogen ions (h+), which will react with the carbonate to form Burning fossil fuels also contributes to this. Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric.. What Happens When Limestone Reacts With Acid.
From www.youtube.com
Acid Metal Reactions YouTube What Happens When Limestone Reacts With Acid When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. Burning fossil fuels also contributes to this. As the limestone dissolves, the rocks will wear. What happens when acid is added to limestone? Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. This. What Happens When Limestone Reacts With Acid.
From fineartamerica.com
Limestone Reacting With Acid Photograph by Andrew Lambert Photography What Happens When Limestone Reacts With Acid When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. Burning fossil fuels also contributes to this. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic. What Happens When Limestone Reacts With Acid.
From www.slideshare.net
REACTION OF METALS WITH ACID What Happens When Limestone Reacts With Acid What happens when acid is added to limestone? In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. As the limestone dissolves, the rocks will wear. This is not very soluble, so rocks don't dissolve very quickly. As acid rain falls to the earth's surface, limestone rocks and limestone components. What Happens When Limestone Reacts With Acid.
From edurev.in
What happens when metal carbonate reacts with acid? Write the chemical What Happens When Limestone Reacts With Acid What happens when acid is added to limestone? Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. In this reaction, the limestone reacts with the acid to produce calcium chloride and. What Happens When Limestone Reacts With Acid.
From yesdirt.com
What Happens When Limestone Is Mixed With Water? Yes Dirt What Happens When Limestone Reacts With Acid This causes the limestone to dissolve. Burning fossil fuels also contributes to this. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. But if you add an acid, you add hydrogen ions (h+), which will react with the carbonate to form As acid rain falls to the earth's surface, limestone. What Happens When Limestone Reacts With Acid.
From dxousntek.blob.core.windows.net
What Happens If You Combine Zinc And Hydrochloric Acid at Debrah Sankey What Happens When Limestone Reacts With Acid Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. As acid rain falls to the. What Happens When Limestone Reacts With Acid.
From www.teachoo.com
Metal compound A reacts with dilute hydrochloric acid to produce What Happens When Limestone Reacts With Acid This is not very soluble, so rocks don't dissolve very quickly. The calcium chloride produced is very soluble in water, and acid can thus speed. Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. What. What Happens When Limestone Reacts With Acid.
From skylargokecox.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid What Happens When Limestone Reacts With Acid Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. This causes the limestone to dissolve. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon. What Happens When Limestone Reacts With Acid.
From aliananewsgaines.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid What Happens When Limestone Reacts With Acid Burning fossil fuels also contributes to this. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. But if you add an acid, you add hydrogen ions (h+), which will react. What Happens When Limestone Reacts With Acid.
From mstimms-gcse.blogspot.com
Ms Timms GCSE Limestone reaction cycle chemistry What Happens When Limestone Reacts With Acid But if you add an acid, you add hydrogen ions (h+), which will react with the carbonate to form The calcium chloride produced is very soluble in water, and acid can thus speed. Burning fossil fuels also contributes to this. This is not very soluble, so rocks don't dissolve very quickly. Limestone is mostly made up of the mineral calcium. What Happens When Limestone Reacts With Acid.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science What Happens When Limestone Reacts With Acid What happens when acid is added to limestone? This is not very soluble, so rocks don't dissolve very quickly. When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. The calcium chloride produced is very soluble in water, and acid can thus speed. This causes the limestone to dissolve. Limestone. What Happens When Limestone Reacts With Acid.
From lexi-bogspotmarsh.blogspot.com
Calcium Carbonate Reacts With Hydrochloric Acid What Happens When Limestone Reacts With Acid Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. Burning fossil fuels also contributes to this. In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. This causes the limestone to dissolve. As acid rain falls to the earth's surface, limestone. What Happens When Limestone Reacts With Acid.
From fphoto.photoshelter.com
science chemistry exothermic reaction limestone hydrochloric acid What Happens When Limestone Reacts With Acid Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. As acid rain falls to the earth's surface, limestone rocks and limestone components in soil will react with the rain, neutralize the acid and dissolve. Limestone is mostly made up of the mineral calcium carbonate (caco3). This causes the limestone to dissolve.. What Happens When Limestone Reacts With Acid.
From www.thenakedscientists.com
What happens when acid reacts with limestone? Science Questions What Happens When Limestone Reacts With Acid Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. Learn how to identify carbonate minerals such as calcite, dolomite, and limestone by placing a drop of hydrochloric. As the limestone dissolves, the rocks will wear. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic. What Happens When Limestone Reacts With Acid.
From cevtgkwv.blob.core.windows.net
What Happens If You Add A Base To An Acid at Gerald Gardella blog What Happens When Limestone Reacts With Acid Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. This is not very soluble,. What Happens When Limestone Reacts With Acid.
From www.youtube.com
GCSE AQA Chemistry Unit 1 (C1) 2.2 Limestone (Part 2) YouTube What Happens When Limestone Reacts With Acid Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. Limestone is. What Happens When Limestone Reacts With Acid.
From blog.thepipingmart.com
What happens when Tungsten Chloride Reacts with Nitric Acid What Happens When Limestone Reacts With Acid This causes the limestone to dissolve. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. This is not very soluble, so rocks don't dissolve very quickly. As acid rain falls. What Happens When Limestone Reacts With Acid.
From fphoto.photoshelter.com
science chemistry exothermic reaction limestone hydrochloric acid What Happens When Limestone Reacts With Acid As acid rain falls to the earth's surface, limestone rocks and limestone components in soil will react with the rain, neutralize the acid and dissolve. What happens when acid is added to limestone? The calcium chloride produced is very soluble in water, and acid can thus speed. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a. What Happens When Limestone Reacts With Acid.
From byjus.com
Ethylamine reacts with nitrous acid to form What Happens When Limestone Reacts With Acid This causes the limestone to dissolve. Limestone is mostly made up of the mineral calcium carbonate (caco3). As the limestone dissolves, the rocks will wear. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. Burning fossil fuels also contributes to this. But if you add an acid, you add hydrogen. What Happens When Limestone Reacts With Acid.
From www.nagwa.com
Question Video Identifying a Sedimentary Rock from Its Appearance What Happens When Limestone Reacts With Acid As acid rain falls to the earth's surface, limestone rocks and limestone components in soil will react with the rain, neutralize the acid and dissolve. Limestone is mostly made up of the mineral calcium carbonate (caco3). The calcium chloride produced is very soluble in water, and acid can thus speed. But if you add an acid, you add hydrogen ions. What Happens When Limestone Reacts With Acid.
From byjus.com
What is the product formed when excess of ethanol reacts with conc What Happens When Limestone Reacts With Acid Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. This is not very soluble, so rocks don't dissolve very quickly. The calcium chloride produced is very soluble in water, and acid can thus speed.. What Happens When Limestone Reacts With Acid.
From slidetodoc.com
Limestone Limestone calcium carbonate WALT Describe what limestone What Happens When Limestone Reacts With Acid Burning fossil fuels also contributes to this. What happens when acid is added to limestone? Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. As the limestone dissolves, the rocks will wear. The. What Happens When Limestone Reacts With Acid.
From www.sciencephoto.com
Limestone Reacts with Acid Stock Image C039/1326 Science Photo What Happens When Limestone Reacts With Acid When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. Limestone is mostly made up of the mineral calcium carbonate (caco3). Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. In this reaction, the limestone reacts with the acid to. What Happens When Limestone Reacts With Acid.
From www.alamy.com
calcium carbonate reacts with dilute hydrochloric acid to produce What Happens When Limestone Reacts With Acid The calcium chloride produced is very soluble in water, and acid can thus speed. What happens when acid is added to limestone? Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water but. This causes the limestone to dissolve. Limestone is mostly made up of the mineral calcium carbonate (caco3). Carbon dioxide from the respiration. What Happens When Limestone Reacts With Acid.
From brainly.in
sodium carbonate reacts with sulphuric acid to form sodium sulphate What Happens When Limestone Reacts With Acid As acid rain falls to the earth's surface, limestone rocks and limestone components in soil will react with the rain, neutralize the acid and dissolve. Limestone is mostly made up of the mineral calcium carbonate (caco3). The calcium chloride produced is very soluble in water, and acid can thus speed. In this reaction, the limestone reacts with the acid to. What Happens When Limestone Reacts With Acid.
From www.youtube.com
What happens when metals react with acid ? Ch. 3 Class10 What Happens When Limestone Reacts With Acid In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. This causes the limestone to dissolve. But if you add an acid, you add hydrogen ions (h+), which will react with the carbonate to form Limestone is a mineral formed of calcium carbonate (caco3), which is slightly soluble in water. What Happens When Limestone Reacts With Acid.
From mavink.com
Sodium Metal Reacts With Hydrochloric Acid What Happens When Limestone Reacts With Acid This causes the limestone to dissolve. When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. The calcium chloride produced is very soluble in water, and acid can thus speed. What happens when acid is added to limestone? Limestone is mostly made up of the mineral calcium carbonate (caco3). Limestone. What Happens When Limestone Reacts With Acid.
From www.youtube.com
What happens when Sodium Hydroxide and Aluminium Reacts What Happens When Limestone Reacts With Acid This is not very soluble, so rocks don't dissolve very quickly. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. In this reaction, the limestone reacts with the acid to produce calcium chloride and carbon dioxide gas, which bubbles off. But if you add an acid, you add hydrogen ions. What Happens When Limestone Reacts With Acid.
From fphoto.photoshelter.com
science chemistry precipitation reaction carbon dioxide Fundamental What Happens When Limestone Reacts With Acid This causes the limestone to dissolve. As acid rain falls to the earth's surface, limestone rocks and limestone components in soil will react with the rain, neutralize the acid and dissolve. As the limestone dissolves, the rocks will wear. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. Limestone is. What Happens When Limestone Reacts With Acid.
From brainly.ph
Limestone reacting with carbonic acid Brainly.ph What Happens When Limestone Reacts With Acid When acid reacts with limestone, a chemical reaction occurs where the acid dissolves the calcium carbonate in the limestone, forming. Carbon dioxide from the respiration of animals (and ourselves) is one cause of increased carbon dioxide in the atmosphere. This causes the limestone to dissolve. Limestone is mostly made up of the mineral calcium carbonate (caco3). But if you add. What Happens When Limestone Reacts With Acid.