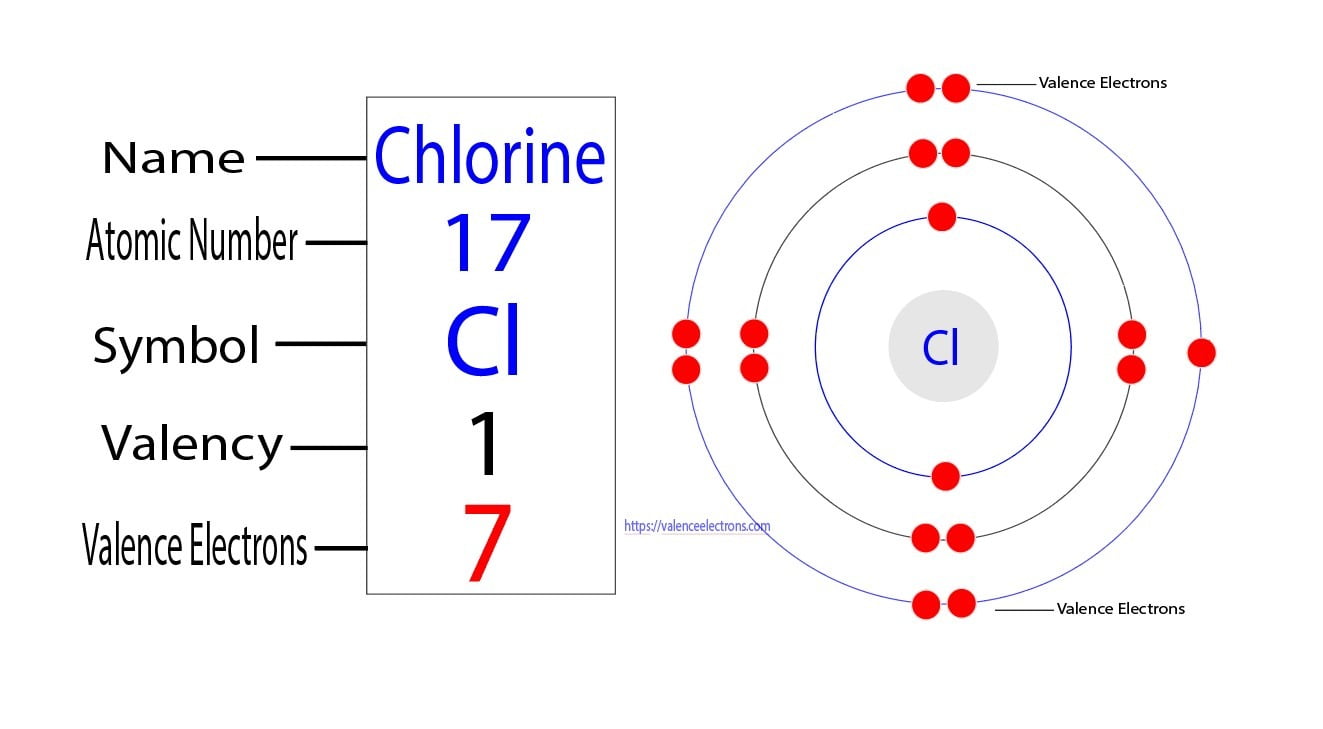
Electrons To Chlorine . This time two chlorine atoms add to a molecule across the. Chlorine can also react with alkenes via the electrophilic addition mechanism. It is an extremely reactive element and a strong oxidising agent: Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. We'll need to know how many sublevel is present. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Electron configuration of chlorine is [ne] 3s2 3p5.
from www.animalia-life.club
This time two chlorine atoms add to a molecule across the. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. It is an extremely reactive element and a strong oxidising agent: Electron configuration of chlorine is [ne] 3s2 3p5. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). We'll need to know how many sublevel is present. Chlorine can also react with alkenes via the electrophilic addition mechanism.
Chloride Ion Number Of Protons And Electrons
Electrons To Chlorine It is an extremely reactive element and a strong oxidising agent: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Chlorine can also react with alkenes via the electrophilic addition mechanism. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. This time two chlorine atoms add to a molecule across the. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). We'll need to know how many sublevel is present. It is an extremely reactive element and a strong oxidising agent: Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form.
From www.nuclear-power.com
Chlorine Atomic Number Atomic Mass Density of Chlorine nuclear Electrons To Chlorine We'll need to know how many sublevel is present. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Chlorine can also react with alkenes via the electrophilic addition mechanism. In order to write the chlorine electron configuration we first need to know the number of electrons for the. Electrons To Chlorine.
From animalia-life.club
Lewis Dot Structure For Chlorine Electrons To Chlorine The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. It is an extremely reactive element and a strong oxidising agent: This time two chlorine atoms add to a molecule across the. Chlorine can also react with alkenes via the electrophilic addition mechanism. Electron configuration of chlorine is [ne] 3s2 3p5.. Electrons To Chlorine.
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron Electrons To Chlorine It is an extremely reactive element and a strong oxidising agent: The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. This time two chlorine atoms add to a molecule across the.. Electrons To Chlorine.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Electrons To Chlorine This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. This time two chlorine atoms add to a molecule across the. We'll need to know how many sublevel is present. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, which means it has 17 protons. Electrons To Chlorine.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Electrons To Chlorine In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17. Electrons To Chlorine.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Electrons To Chlorine The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. This time two chlorine atoms add to a molecule across the. It is an extremely reactive element and a strong oxidising agent: In order to write the chlorine electron configuration we first need to know the number of electrons for the. Electrons To Chlorine.
From brainly.in
draw atomic structure of chlorine Brainly.in Electrons To Chlorine This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Chlorine can also react with alkenes via the electrophilic addition mechanism. Electron configuration of chlorine is [ne] 3s2 3p5. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. It. Electrons To Chlorine.
From animalia-life.club
Lewis Dot Structure For Chlorine Electrons To Chlorine Chlorine can also react with alkenes via the electrophilic addition mechanism. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Electron configuration of chlorine is [ne] 3s2 3p5. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded. Electrons To Chlorine.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Electrons To Chlorine This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Chlorine can also react with alkenes via the electrophilic addition mechanism. It is an extremely reactive element and a strong oxidising agent: Electron configuration of chlorine is [ne] 3s2 3p5. In order to write the chlorine electron configuration we first need. Electrons To Chlorine.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Electrons To Chlorine The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. We'll need to know how many sublevel is present. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Electron configuration of chlorine is [ne] 3s2 3p5. In order to. Electrons To Chlorine.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Electrons To Chlorine The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. Chlorine can also react with alkenes via the electrophilic addition mechanism. This electron configuration calculator will instantly show you the. Electrons To Chlorine.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Electrons To Chlorine The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. We'll need to know how many sublevel is present. Electron configuration of chlorine is [ne] 3s2 3p5. In order to write the. Electrons To Chlorine.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Electrons To Chlorine This time two chlorine atoms add to a molecule across the. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. It is an extremely reactive element and a strong oxidising agent: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any. Electrons To Chlorine.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Electrons To Chlorine Chlorine can also react with alkenes via the electrophilic addition mechanism. This time two chlorine atoms add to a molecule across the. We'll need to know how many sublevel is present. Electron configuration of chlorine is [ne] 3s2 3p5. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom. Electrons To Chlorine.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Electrons To Chlorine In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. This time two chlorine atoms add to a molecule across the. We'll need to. Electrons To Chlorine.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Electrons To Chlorine Electron configuration of chlorine is [ne] 3s2 3p5. This time two chlorine atoms add to a molecule across the. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. Chlorine. Electrons To Chlorine.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Electrons To Chlorine It is an extremely reactive element and a strong oxidising agent: We'll need to know how many sublevel is present. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. This time two chlorine atoms add to a molecule across the. In order to write the chlorine electron configuration. Electrons To Chlorine.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Electrons To Chlorine We'll need to know how many sublevel is present. Electron configuration of chlorine is [ne] 3s2 3p5. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. It is an extremely reactive element and a strong oxidising agent: Chlorine has an atomic number of 17, which means it has 17 protons. Electrons To Chlorine.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of Electrons To Chlorine In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Chlorine can also react with alkenes via the electrophilic addition mechanism. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Chlorine has an atomic number. Electrons To Chlorine.
From www.youtube.com
Chlorine Electron Configuration YouTube Electrons To Chlorine Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). This electron configuration calculator will instantly show you the distribution of electrons in the. Electrons To Chlorine.
From animalia-life.club
Lewis Dot Structure For Chlorine Electrons To Chlorine Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). This electron configuration calculator will instantly show you the distribution of electrons in the. Electrons To Chlorine.
From material-properties.org
Chlorine Protons Neutrons Electrons Electron Configuration Electrons To Chlorine This time two chlorine atoms add to a molecule across the. It is an extremely reactive element and a strong oxidising agent: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy.. Electrons To Chlorine.
From www.istockphoto.com
Atom Of Chlorine With Core And 17 Electrons On White Stock Photo Electrons To Chlorine It is an extremely reactive element and a strong oxidising agent: Chlorine can also react with alkenes via the electrophilic addition mechanism. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element.. Electrons To Chlorine.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Electrons To Chlorine Chlorine can also react with alkenes via the electrophilic addition mechanism. Electron configuration of chlorine is [ne] 3s2 3p5. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. We'll need to. Electrons To Chlorine.
From www.chemistrystudent.com
Covalent Bonding (ALevel) ChemistryStudent Electrons To Chlorine This time two chlorine atoms add to a molecule across the. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). We'll need to know how many sublevel is present. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals. Electrons To Chlorine.
From sciencenotes.org
Chlorine Facts Electrons To Chlorine This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. It is an extremely reactive element and a strong oxidising agent: Chlorine can also react with alkenes via the electrophilic addition mechanism.. Electrons To Chlorine.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Electrons To Chlorine In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). We'll need to know how many sublevel is present. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. The atomic structure of chlorine consists of. Electrons To Chlorine.
From www.dreamstime.com
Atom of Chlorine with Detailed Core and Its 17 Electrons with Atoms Electrons To Chlorine Chlorine can also react with alkenes via the electrophilic addition mechanism. This time two chlorine atoms add to a molecule across the. Electron configuration of chlorine is [ne] 3s2 3p5. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). It is an extremely reactive. Electrons To Chlorine.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Electrons To Chlorine The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. We'll need to know how many sublevel is present. Chlorine has an atomic number of 17, which means it has 17 protons. Electrons To Chlorine.
From littleeagles.edu.vn
How To Find The Protons, Neutrons And Electrons For Chlorine? Electrons To Chlorine This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Electron configuration of chlorine is [ne] 3s2 3p5. It is an extremely reactive element and a strong oxidising agent: This time two chlorine atoms add to a molecule across the. We'll need to know how many sublevel is present. Chlorine can. Electrons To Chlorine.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Electrons To Chlorine Chlorine can also react with alkenes via the electrophilic addition mechanism. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. It is an extremely reactive element and a strong oxidising agent: This time two chlorine atoms add to a molecule across the. We'll need to know how many sublevel is. Electrons To Chlorine.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Electrons To Chlorine Electron configuration of chlorine is [ne] 3s2 3p5. The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by three energy. We'll need to know how many sublevel is present. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. It is an extremely reactive. Electrons To Chlorine.
From pixels.com
Chlorine Electron Configuration Photograph by Electrons To Chlorine It is an extremely reactive element and a strong oxidising agent: This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element. Chlorine can also react with alkenes via the electrophilic addition mechanism. We'll need to know how many sublevel is present. Electron configuration of chlorine is [ne] 3s2 3p5. In order. Electrons To Chlorine.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Electrons To Chlorine This time two chlorine atoms add to a molecule across the. We'll need to know how many sublevel is present. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. It is an extremely reactive element and a strong oxidising agent: Chlorine can also react with alkenes via the. Electrons To Chlorine.
From stock.adobe.com
Periodic Table of the Elements, Shell Structure of Chlorine Cl Electrons To Chlorine This time two chlorine atoms add to a molecule across the. Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. It is an extremely reactive element and a strong oxidising agent: The atomic structure of chlorine consists of a nucleus containing 17 protons and 17 neutrons, surrounded by. Electrons To Chlorine.