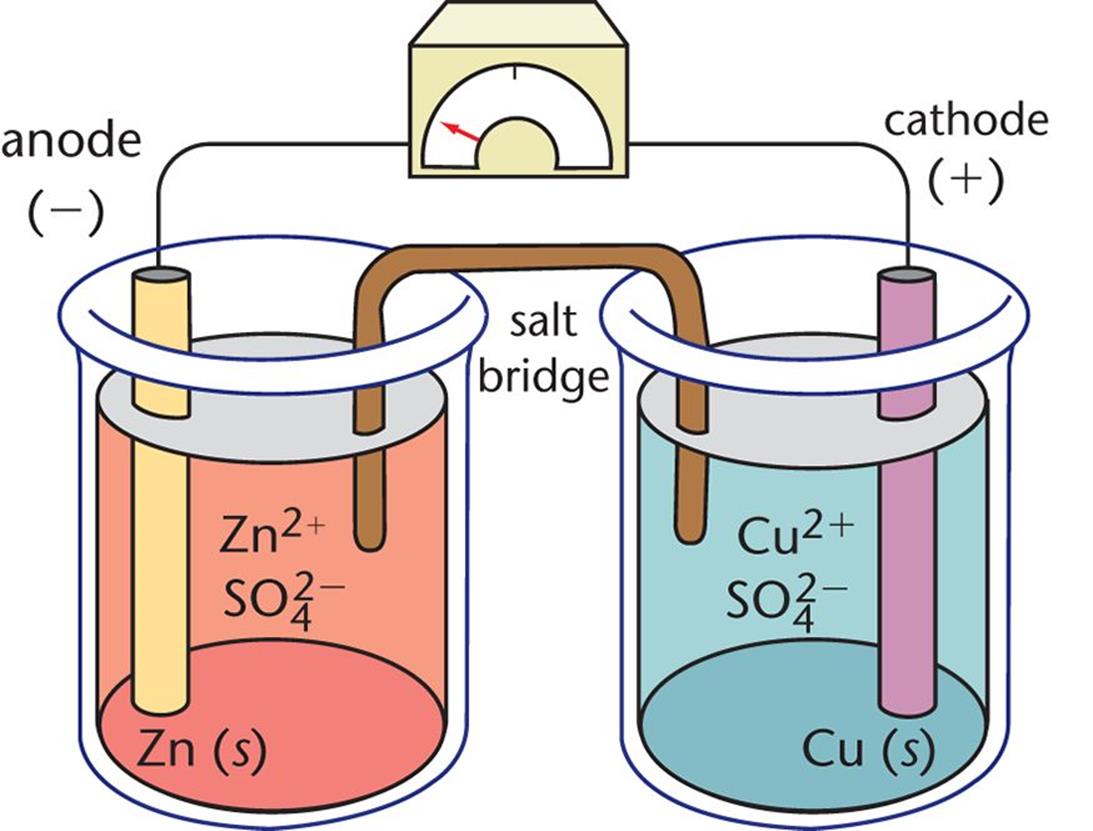
Voltaic Cell Is Set . A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Voltaic cells are typically used as a source of electrical power. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. By their nature, they produce direct current. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A battery is a set of voltaic cells that are connected in parallel.
from schoolbag.info
Voltaic cells are typically used as a source of electrical power. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A battery is a set of voltaic cells that are connected in parallel. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. By their nature, they produce direct current. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the.
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and
Voltaic Cell Is Set Voltaic cells are typically used as a source of electrical power. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. By their nature, they produce direct current. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A battery is a set of voltaic cells that are connected in parallel. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. Voltaic cells are typically used as a source of electrical power.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Voltaic Cell Is Set By their nature, they produce direct current. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg <. Voltaic Cell Is Set.
From courses.lumenlearning.com
17.2 Galvanic Cells (Voltaic Cells) General College Chemistry II Voltaic Cell Is Set A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. Voltaic cells are typically used as a source of electrical power. By their nature, they produce direct current. A battery is a set of voltaic cells that are connected in parallel. A galvanic (voltaic) cell uses the energy released during a spontaneous redox. Voltaic Cell Is Set.
From www.youtube.com
9.2 Voltaic cells (SL) YouTube Voltaic Cell Is Set A battery is a set of voltaic cells that are connected in parallel. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. The galvanic cell, or called voltaic cell,. Voltaic Cell Is Set.
From wisc.pb.unizin.org
D39.4 Introduction to Voltaic Cells Chemistry 109 Fall 2021 Voltaic Cell Is Set The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. Voltaic cells are typically used as a source of electrical power. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. By their nature, they produce direct current. A galvanic (voltaic) cell. Voltaic Cell Is Set.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Voltaic Cell Is Set By their nature, they produce direct current. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A battery is a set of voltaic cells that are connected in parallel.. Voltaic Cell Is Set.
From wisc.pb.unizin.org
Day 39 Voltaic Cells Chemistry 109 Voltaic Cell Is Set Voltaic cells are typically used as a source of electrical power. A battery is a set of voltaic cells that are connected in parallel. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction. Voltaic Cell Is Set.
From overallscience.com
Differences between electrolytic cell and voltaic cell Overall Science Voltaic Cell Is Set The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)). Voltaic Cell Is Set.
From www.chegg.com
Solved A voltaic cell is setup with a gallium electrode in Voltaic Cell Is Set A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A battery is a set of voltaic cells that are connected in parallel. A voltaic cell. Voltaic Cell Is Set.
From mavink.com
Simple Voltaic Cell Voltaic Cell Is Set Voltaic cells are typically used as a source of electrical power. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A battery is a set of voltaic cells that are connected in parallel. By their nature, they produce direct current. A galvanic (voltaic) cell uses the energy released. Voltaic Cell Is Set.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Voltaic Cell Is Set A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Voltaic cells are typically used as a source of electrical power. The galvanic cell, or called voltaic cell, is an electrochemical cell. Voltaic Cell Is Set.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 11 simple voltaic cell Voltaic Cell Is Set The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)). Voltaic Cell Is Set.
From overallscience.com
Voltaic Cell, Its Construction and Defects Overall Science Voltaic Cell Is Set A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. By their nature, they produce direct current. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A galvanic (voltaic) cell uses the energy released during a spontaneous. Voltaic Cell Is Set.
From www.slideserve.com
PPT Chemistry 100 Chapter 20 PowerPoint Presentation, free download Voltaic Cell Is Set Voltaic cells are typically used as a source of electrical power. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. By their nature, they produce direct current. A galvanic (voltaic) cell. Voltaic Cell Is Set.
From www.transformationtutoring.com
The diagram and ionic equation below represent an operating voltaic cell. Voltaic Cell Is Set The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. By their nature, they produce direct current. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses. Voltaic Cell Is Set.
From diagramlibraryclopped.z19.web.core.windows.net
Diagram Of Voltaic Cell Voltaic Cell Is Set By their nature, they produce direct current. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an. Voltaic Cell Is Set.
From www.chegg.com
Solved A voltaic cell is set up as shown below. The silver Voltaic Cell Is Set A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A battery is a set of voltaic cells that are connected in parallel. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. By their nature, they produce direct current. A galvanic. Voltaic Cell Is Set.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Voltaic Cell Is Set By their nature, they produce direct current. Voltaic cells are typically used as a source of electrical power. A battery is a set of voltaic cells that are connected in parallel. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell (also known as a galvanic. Voltaic Cell Is Set.
From byjus.com
The electrolyte of simple voltaic cell is Voltaic Cell Is Set Voltaic cells are typically used as a source of electrical power. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. The galvanic cell, or called voltaic cell, is an. Voltaic Cell Is Set.
From newlasertagatlanta.blogspot.com
39 label the diagram according to the components and processes of a Voltaic Cell Is Set The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical. Voltaic Cell Is Set.
From www.chegg.com
Solved A voltaic cell is made with silver, silver nitrate, Voltaic Cell Is Set A battery is a set of voltaic cells that are connected in parallel. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A galvanic cell is a. Voltaic Cell Is Set.
From www.peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Voltaic Cell Is Set A battery is a set of voltaic cells that are connected in parallel. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A galvanic (voltaic) cell uses the energy released during. Voltaic Cell Is Set.
From drcalef.com
A Voltaic Cell Voltaic Cell Is Set A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. By their nature, they produce direct current. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and. Voltaic Cell Is Set.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5570772 Voltaic Cell Is Set A battery is a set of voltaic cells that are connected in parallel. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. Voltaic cells are typically used as a source of electrical power. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and. Voltaic Cell Is Set.
From chem2u.blogspot.com
chem2U Simple Voltaic Cell Voltaic Cell Is Set A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. Voltaic cells are typically used as a source of electrical power. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. By their nature, they produce direct current. A battery. Voltaic Cell Is Set.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Voltaic Cell Is Set A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A battery is a set of voltaic cells that are connected in parallel. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A voltaic cell. Voltaic Cell Is Set.
From theedge.com.hk
Chemistry How To Electrochemical Cell The Edge Voltaic Cell Is Set The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge. Voltaic Cell Is Set.
From saylordotorg.github.io
Describing Electrochemical Cells Voltaic Cell Is Set A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. Voltaic cells are typically used as a source of electrical power. By their nature, they produce direct current. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A galvanic cell is. Voltaic Cell Is Set.
From www.chegg.com
Solved A voltaic cell is set up as follows. The Voltaic Cell Is Set The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. By their nature, they produce direct current. A battery is a set of voltaic cells that are connected in parallel. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A voltaic. Voltaic Cell Is Set.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Voltaic Cell Is Set The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from the. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Voltaic cells are typically used as a source of electrical power. A galvanic cell is a cell where. Voltaic Cell Is Set.
From www.carolina.com
Voltaic Cell Set, Student Voltaic Cell Is Set A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and a salt bridge produce electric energy. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A battery is a set of voltaic cells that are connected in parallel. By their nature, they produce. Voltaic Cell Is Set.
From www.youtube.com
Explain the general set up of a voltaic cell YouTube Voltaic Cell Is Set By their nature, they produce direct current. Voltaic cells are typically used as a source of electrical power. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A battery is a set of voltaic cells that are connected in parallel. A galvanic cell is a cell where chemical. Voltaic Cell Is Set.
From www.electroniclinic.com
What is battery? Types of battery, Primary and Secondary cells Voltaic Cell Is Set A battery is a set of voltaic cells that are connected in parallel. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. Voltaic cells are typically used as a source of electrical power. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected through an electrolyte and. Voltaic Cell Is Set.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Voltaic Cell Is Set A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. By their nature, they produce direct current. A galvanic cell is a cell where chemical reactions between dissimilar conductors connected. Voltaic Cell Is Set.
From quizlet.com
Voltaic Cell Diagram Diagram Quizlet Voltaic Cell Is Set Voltaic cells are typically used as a source of electrical power. By their nature, they produce direct current. A battery is a set of voltaic cells that are connected in parallel. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell is an electrochemical cell that uses a. Voltaic Cell Is Set.
From jackwestin.com
Galvanic Or Voltaic Cells Electrochemistry MCAT Content Voltaic Cell Is Set Voltaic cells are typically used as a source of electrical power. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A battery is a set of voltaic cells that are connected in parallel. The galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy. Voltaic Cell Is Set.