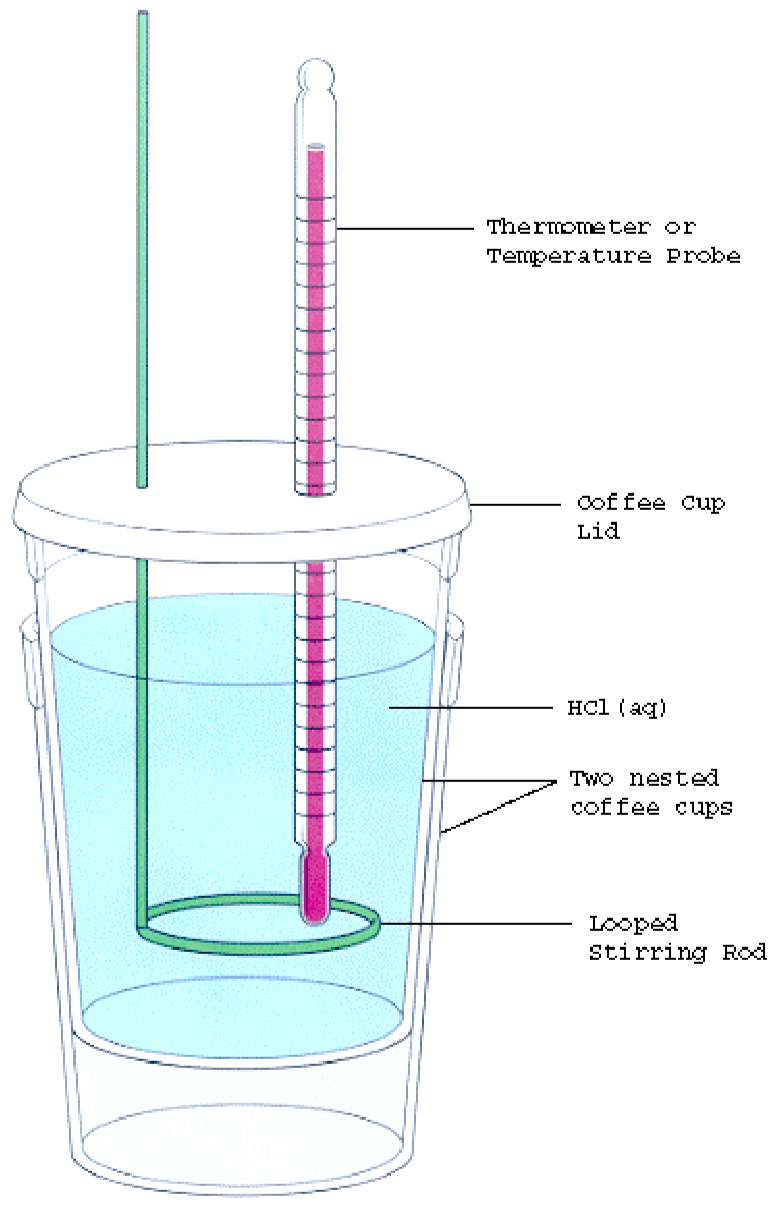
Calorimetry Procedure . a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. calculate and interpret heat and related properties using typical calorimetry data. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. One technique we can use to measure the. a calorimeter is a device used to determine heat flow during a chemical or physical change. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. in this lab, you will do two classic calorimetry experiments: Measuring the latent heat of fusion of water, and measuring the specific heat capacities.
from chem.libretexts.org
one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. in this lab, you will do two classic calorimetry experiments: Calorimetry is used to measure. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. One technique we can use to measure the. calculate and interpret heat and related properties using typical calorimetry data. a calorimeter is a device used to determine heat flow during a chemical or physical change.
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts
Calorimetry Procedure in this lab, you will do two classic calorimetry experiments: a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. in this lab, you will do two classic calorimetry experiments: calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is used to measure. a calorimeter is a device used to determine heat flow during a chemical or physical change. One technique we can use to measure the. calculate and interpret heat and related properties using typical calorimetry data.
From www.studypool.com
SOLUTION Activity 3 calorimetry procedure Studypool Calorimetry Procedure calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. One technique we can use to measure the. Calorimetry is used to measure. Measuring the latent heat of fusion of water,. Calorimetry Procedure.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3167873 Calorimetry Procedure calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. One technique we can use to measure the. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. a calorimeter is a device used to measure the amount of heat. Calorimetry Procedure.
From www.youtube.com
M6 Differential Scanning Calorimetry Review of Operating Principles Calorimetry Procedure Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. a calorimeter is a device used to determine heat flow during a chemical or physical change. . Calorimetry Procedure.
From dxoqgiaho.blob.core.windows.net
Bomb Calorimeter Enthalpy Of Combustion at Harold Shaner blog Calorimetry Procedure Calorimetry is used to measure. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Calorimetry Procedure.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Procedure one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. One technique we can use to measure the. calculate and interpret heat and related properties using typical calorimetry. Calorimetry Procedure.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID765480 Calorimetry Procedure one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. in this lab, you will do two classic calorimetry experiments: a calorimeter is a. Calorimetry Procedure.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Procedure calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calculate and interpret heat and related properties using typical calorimetry data. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the. in. Calorimetry Procedure.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Procedure Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. Calorimetry is used to measure. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. calculate and interpret heat and related properties using typical calorimetry data. one technique we can use to measure. Calorimetry Procedure.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Procedure Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. calculate and interpret heat and related properties using typical calorimetry data. in this lab, you will do two classic calorimetry experiments: calorimetry. Calorimetry Procedure.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Calorimetry Procedure One technique we can use to measure the. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Measuring the latent heat of fusion of water, and measuring the. Calorimetry Procedure.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Procedure Calorimetry is used to measure. calculate and interpret heat and related properties using typical calorimetry data. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. One technique we can use to measure the. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Procedure.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Procedure calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. in this lab, you will do two classic calorimetry experiments: Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. one technique we can use to measure the amount. Calorimetry Procedure.
From www.researchgate.net
Experimental procedure and raw results of calorimetry experiments. a Calorimetry Procedure one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. in this lab, you will do two classic calorimetry experiments: Calorimetry is used to measure. a calorimeter is a device used to determine heat flow during a chemical or physical change. calculate and interpret. Calorimetry Procedure.
From www.studypool.com
SOLUTION Activity 3 calorimetry procedure Studypool Calorimetry Procedure calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is used to measure. a calorimeter is a device used to determine heat flow during a chemical or physical change. in this lab, you will do two classic calorimetry experiments: one technique we can use to measure the amount of heat involved in a. Calorimetry Procedure.
From saylordotorg.github.io
Calorimetry Calorimetry Procedure Measuring the latent heat of fusion of water, and measuring the specific heat capacities. in this lab, you will do two classic calorimetry experiments: calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimetry Procedure.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Procedure Measuring the latent heat of fusion of water, and measuring the specific heat capacities. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to determine heat flow during a chemical or physical change. Determination of the heat capacity of the calorimeter (calorimeter. Calorimetry Procedure.
From slideplayer.com
Specific Heat Capacity & Calorimetry ppt download Calorimetry Procedure one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. a calorimeter. Calorimetry Procedure.
From www.studypool.com
SOLUTION Activity 3 calorimetry procedure Studypool Calorimetry Procedure Measuring the latent heat of fusion of water, and measuring the specific heat capacities. a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the. Calorimetry. Calorimetry Procedure.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimetry Procedure calculate and interpret heat and related properties using typical calorimetry data. in this lab, you will do two classic calorimetry experiments: Calorimetry is used to measure. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat. Calorimetry Procedure.
From www.youtube.com
Thermophysical characterization Bomb calorimeter (Standard Operating Calorimetry Procedure a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. a calorimeter is a device used to determine heat flow during a chemical or physical change. calculate. Calorimetry Procedure.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Procedure Measuring the latent heat of fusion of water, and measuring the specific heat capacities. Calorimetry is used to measure. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. a calorimeter is a device used to determine heat flow during a chemical or physical change. calculate and. Calorimetry Procedure.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Calorimetry Procedure one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in this lab, you will do two classic calorimetry experiments: One technique we can use to. Calorimetry Procedure.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Procedure calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to determine heat flow during a chemical or physical change. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a. Calorimetry Procedure.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Procedure One technique we can use to measure the. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. in this lab, you will do two classic calorimetry experiments: Calorimetry is used to measure. . Calorimetry Procedure.
From www.slideshare.net
2 pa32 bomb calorimeter procedure Calorimetry Procedure One technique we can use to measure the. calculate and interpret heat and related properties using typical calorimetry data. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to determine heat flow during a chemical or physical change. Calorimetry is used to measure.. Calorimetry Procedure.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Procedure Measuring the latent heat of fusion of water, and measuring the specific heat capacities. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a. Calorimetry Procedure.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimetry Procedure in this lab, you will do two classic calorimetry experiments: One technique we can use to measure the. a calorimeter is a device used to determine heat flow during a chemical or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to. Calorimetry Procedure.
From studyfinder.org
Unveiling the Secrets of Experiment 25 Calorimetry Prelab Answers Calorimetry Procedure a calorimeter is a device used to determine heat flow during a chemical or physical change. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. Calorimetry is used to measure. in this lab, you will do two classic calorimetry experiments: a calorimeter is a device used to measure the amount of heat. Calorimetry Procedure.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimetry Procedure calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is used to measure. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to determine heat flow during a chemical or physical change. One technique we can use to. Calorimetry Procedure.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Procedure a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. calculate and interpret heat and related properties using typical calorimetry. Calorimetry Procedure.
From www.scribd.com
Calorimetry Procedure PDF Chemical Reactions Combustion Calorimetry Procedure One technique we can use to measure the. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. calculate and interpret heat and related properties using typical calorimetry data. Determination of the. Calorimetry Procedure.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Calorimetry Procedure one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Determination of the heat capacity of the calorimeter (calorimeter constant) the calorimeter consists of an insulated cup covered with a cardboard. calorimetry is the process of measuring the amount of heat released or absorbed during a. Calorimetry Procedure.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Calorimetry Procedure Calorimetry is used to measure. One technique we can use to measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to determine heat flow during a chemical or physical change. one technique we can use to measure the amount. Calorimetry Procedure.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Procedure calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate and interpret heat and related properties using typical calorimetry data. Measuring the latent heat of fusion of water, and measuring. Calorimetry Procedure.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Procedure calculate and interpret heat and related properties using typical calorimetry data. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities. in this lab, you will do two classic calorimetry experiments:. Calorimetry Procedure.