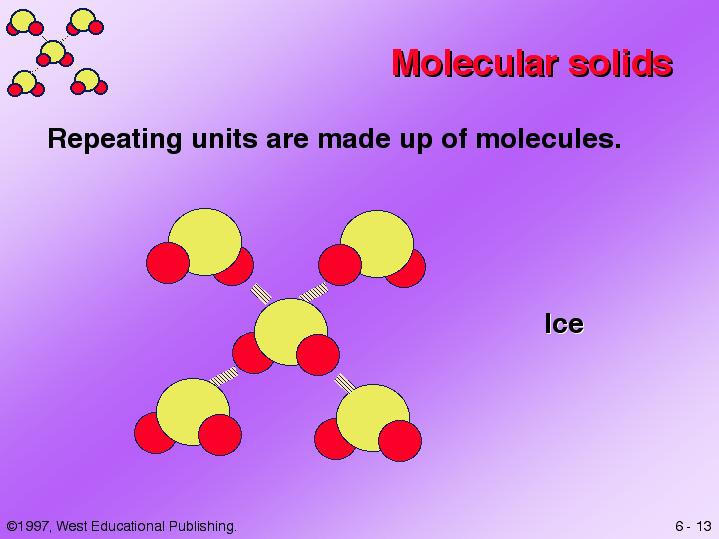
Is Cu A Molecular Solid . Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. Sources, facts, uses, scarcity (sri), podcasts,. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. The dipole forces are weaker than ionic or covalent bonds. a molecular solid is composed of molecules held together by van der waals forces. To understand the correlation between bonding and the properties of solids. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. Its properties are dictated by the weak nature of these intermolecular forces.
from www.chem.uwec.edu
The dipole forces are weaker than ionic or covalent bonds. Its properties are dictated by the weak nature of these intermolecular forces. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. To understand the correlation between bonding and the properties of solids. a molecular solid is composed of molecules held together by van der waals forces. Sources, facts, uses, scarcity (sri), podcasts,. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules.
Molecular solids
Is Cu A Molecular Solid Its properties are dictated by the weak nature of these intermolecular forces. Sources, facts, uses, scarcity (sri), podcasts,. The dipole forces are weaker than ionic or covalent bonds. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. Its properties are dictated by the weak nature of these intermolecular forces. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. a molecular solid is composed of molecules held together by van der waals forces. To understand the correlation between bonding and the properties of solids. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators.
From schoolbag.info
CLASSIFICATIONS OF SOLIDS SOLIDS AND MODERN MATERIALS CHEMISTRY THE Is Cu A Molecular Solid a molecular solid is composed of molecules held together by van der waals forces. Sources, facts, uses, scarcity (sri), podcasts,. To understand the correlation between bonding and the properties of solids. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. The strengths of the attractive forces between the units present in different crystals vary. Is Cu A Molecular Solid.
From www.app.formative.com
Types of Solids Caroline Duesing Library Formative Is Cu A Molecular Solid To understand the correlation between bonding and the properties of solids. Sources, facts, uses, scarcity (sri), podcasts,. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral. Is Cu A Molecular Solid.
From www.meritnation.com
Explain the cubic and hexagonal close packing in solids what is the Is Cu A Molecular Solid The dipole forces are weaker than ionic or covalent bonds. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. a molecular solid is composed of molecules held together by van der waals forces. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather. Is Cu A Molecular Solid.
From animalia-life.club
Covalent Network Solids Is Cu A Molecular Solid Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. Sources, facts, uses, scarcity (sri), podcasts,. a molecular solid is composed of molecules held together by van der waals forces. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Molecular. Is Cu A Molecular Solid.
From www.chemtube3d.com
Copper (I) Oxide Cu2O Is Cu A Molecular Solid Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. The dipole forces are weaker than ionic or covalent bonds. To understand the correlation between bonding and the properties of solids. a molecular solid is composed of molecules held together by van der waals forces. The strengths of the. Is Cu A Molecular Solid.
From ecampusontario.pressbooks.pub
10.5 The Solid State of Matter Chemistry Is Cu A Molecular Solid The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. Its properties are dictated by the weak nature of these intermolecular forces. Sources, facts, uses, scarcity. Is Cu A Molecular Solid.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Is Cu A Molecular Solid Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. a molecular solid is composed of molecules held together by van der waals forces. The dipole forces are weaker than ionic or covalent bonds. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather. Is Cu A Molecular Solid.
From saylordotorg.github.io
Solids and Liquids Is Cu A Molecular Solid The dipole forces are weaker than ionic or covalent bonds. Its properties are dictated by the weak nature of these intermolecular forces. Sources, facts, uses, scarcity (sri), podcasts,. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. a molecular solid is. Is Cu A Molecular Solid.
From soyungringo.blogspot.com
metallic bonding occurs between atoms of best tricktaking card games Is Cu A Molecular Solid To understand the correlation between bonding and the properties of solids. a molecular solid is composed of molecules held together by van der waals forces. The dipole forces are weaker than ionic or covalent bonds. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. The strengths of the. Is Cu A Molecular Solid.
From www.vrogue.co
Facts About Copper Meanings Properties And Benefits I vrogue.co Is Cu A Molecular Solid a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Molecular solids, such as ice, sucrose (table sugar), and. Is Cu A Molecular Solid.
From www.youtube.com
Electron Configuration for Cu, Cu+, and Cu2+ (Copper and Copper Ions Is Cu A Molecular Solid The dipole forces are weaker than ionic or covalent bonds. Sources, facts, uses, scarcity (sri), podcasts,. Its properties are dictated by the weak nature of these intermolecular forces. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. To understand the correlation between. Is Cu A Molecular Solid.
From www.slideserve.com
PPT Types of Solids PowerPoint Presentation, free download ID2940841 Is Cu A Molecular Solid To understand the correlation between bonding and the properties of solids. Sources, facts, uses, scarcity (sri), podcasts,. Its properties are dictated by the weak nature of these intermolecular forces. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. The dipole forces are. Is Cu A Molecular Solid.
From www.chegg.com
Solved Which substance is considered a molecular solid? Is Cu A Molecular Solid Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. Sources, facts, uses, scarcity (sri), podcasts,. Its properties are dictated by the weak nature of these intermolecular forces. The strengths of the attractive forces between the. Is Cu A Molecular Solid.
From www.numerade.com
SOLVED Solid copper reacts with solid sulfur(S8) to form solid copper Is Cu A Molecular Solid Sources, facts, uses, scarcity (sri), podcasts,. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Molecular solids are. Is Cu A Molecular Solid.
From www.chem.uwec.edu
Molecular solids Is Cu A Molecular Solid The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. a molecular solid is composed of molecules held together by van der waals forces. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. To. Is Cu A Molecular Solid.
From www.researchgate.net
The molecular structure of the Cu (II) complex Download Scientific Is Cu A Molecular Solid Its properties are dictated by the weak nature of these intermolecular forces. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. The dipole forces are weaker than ionic or covalent bonds. a molecular solid is a type of solid in which molecules are held together by van der. Is Cu A Molecular Solid.
From thechemistrynotes.com
Copper (Cu) Element Important PhysicalChemical Properties, Is Cu A Molecular Solid The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. The dipole forces are weaker than ionic or covalent bonds. Its properties are dictated by the weak nature of these intermolecular forces. a molecular solid is a type of solid in which molecules are held. Is Cu A Molecular Solid.
From dxoyvzvii.blob.core.windows.net
Copper Symbol Periodic Table at Jean Clarke blog Is Cu A Molecular Solid a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. To understand the correlation between bonding and the properties of solids. Its properties are dictated by the weak nature. Is Cu A Molecular Solid.
From www.alamy.com
Copper Atom High Resolution Stock Photography and Images Alamy Is Cu A Molecular Solid a molecular solid is composed of molecules held together by van der waals forces. To understand the correlation between bonding and the properties of solids. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. Sources, facts, uses, scarcity (sri), podcasts,. Molecular. Is Cu A Molecular Solid.
From www.slideserve.com
PPT Molecular Solids PowerPoint Presentation, free download ID1988592 Is Cu A Molecular Solid The dipole forces are weaker than ionic or covalent bonds. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. To understand the correlation between bonding and the properties of solids. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or. Is Cu A Molecular Solid.
From saylordotorg.github.io
Solids Is Cu A Molecular Solid To understand the correlation between bonding and the properties of solids. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. a molecular solid is composed of molecules held together by. Is Cu A Molecular Solid.
From www.slideserve.com
PPT Chapter 1 Crystal Structure PowerPoint Presentation, free Is Cu A Molecular Solid To understand the correlation between bonding and the properties of solids. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Sources, facts, uses, scarcity (sri), podcasts,. a molecular solid is composed of molecules held together by van der waals forces. Molecular solids, such as. Is Cu A Molecular Solid.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with Is Cu A Molecular Solid a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. Sources, facts, uses, scarcity (sri), podcasts,. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. The dipole forces are weaker than ionic or covalent bonds. a molecular. Is Cu A Molecular Solid.
From www.w3schools.blog
Ionic Solids W3schools Is Cu A Molecular Solid The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. To understand the correlation between bonding and the properties of solids. The dipole forces are weaker than ionic or covalent bonds. Its properties are dictated by the weak nature of these intermolecular forces. a molecular. Is Cu A Molecular Solid.
From dxomqdixz.blob.core.windows.net
Is Brass A Molecular Solid at Robert Martinez blog Is Cu A Molecular Solid Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. To understand the correlation between bonding and the properties of solids. a molecular solid is composed of molecules held together by. Is Cu A Molecular Solid.
From www.youtube.com
Molecular Solids YouTube Is Cu A Molecular Solid Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent. Is Cu A Molecular Solid.
From www.vectorstock.com
Symbol and electron diagram for copper Royalty Free Vector Is Cu A Molecular Solid Its properties are dictated by the weak nature of these intermolecular forces. a molecular solid is composed of molecules held together by van der waals forces. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. Molecular solids are soft, often volatile,. Is Cu A Molecular Solid.
From diagramlistreporting.z14.web.core.windows.net
Diagram Of Particles In Solid Liquid And Gas Is Cu A Molecular Solid The dipole forces are weaker than ionic or covalent bonds. a molecular solid is composed of molecules held together by van der waals forces. To understand the correlation between bonding and the properties of solids. Sources, facts, uses, scarcity (sri), podcasts,. Its properties are dictated by the weak nature of these intermolecular forces. Molecular solids are soft, often volatile,. Is Cu A Molecular Solid.
From thechemistrynotes.com
Ionic Solids Structure, Formation, Properties Is Cu A Molecular Solid The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Sources, facts, uses, scarcity (sri), podcasts,. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. To understand the correlation between bonding and the properties of solids. a molecular solid is. Is Cu A Molecular Solid.
From www.slideserve.com
PPT Molecular Solids PowerPoint Presentation, free download ID1988592 Is Cu A Molecular Solid The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. The dipole forces are weaker than ionic or covalent bonds. Sources, facts, uses, scarcity (sri), podcasts,. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules.. Is Cu A Molecular Solid.
From www.youtube.com
Ionic Solids, Molecular Solids, Metallic Solids, Network Covalent Is Cu A Molecular Solid a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Sources, facts, uses, scarcity (sri), podcasts,. Its properties are. Is Cu A Molecular Solid.
From www.youtube.com
Molecular Solids 1st year Chemistry swap education portal YouTube Is Cu A Molecular Solid a molecular solid is composed of molecules held together by van der waals forces. To understand the correlation between bonding and the properties of solids. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. The dipole forces are weaker than ionic or covalent bonds. Its properties are dictated. Is Cu A Molecular Solid.
From www.youtube.com
Covalent Network Solids YouTube Is Cu A Molecular Solid To understand the correlation between bonding and the properties of solids. a molecular solid is composed of molecules held together by van der waals forces. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. The strengths of the attractive forces between the units present in different crystals vary. Is Cu A Molecular Solid.
From www.slideserve.com
PPT Molecular Solids PowerPoint Presentation, free download ID1988592 Is Cu A Molecular Solid The dipole forces are weaker than ionic or covalent bonds. Sources, facts, uses, scarcity (sri), podcasts,. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent bonds. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. The strengths of. Is Cu A Molecular Solid.
From en.wikipedia.org
Copper Wikipedia Is Cu A Molecular Solid Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in figure 6, are composed of neutral molecules. a molecular solid is a type of solid in which molecules are held together by van der waals forces rather than by ionic or covalent. Is Cu A Molecular Solid.