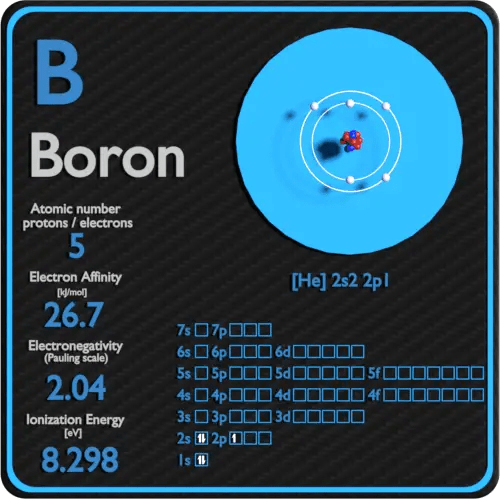
What Is The Difference Between Boron 10 And Boron 11 . After neutron capture, the 10 b (n,α ) to. Boron is an element with two stable isotopes: These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. There are 13 radioisotopes that have been. The atomic mass of boron is 10.81 u. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron.
from material-properties.org
Boron is an element with two stable isotopes: After neutron capture, the 10 b (n,α ) to. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. There are 13 radioisotopes that have been. The atomic mass of boron is 10.81 u.
Boron Periodic Table and Atomic Properties
What Is The Difference Between Boron 10 And Boron 11 These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. There are 13 radioisotopes that have been. After neutron capture, the 10 b (n,α ) to. Boron is an element with two stable isotopes: The atomic mass of boron is 10.81 u. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei.
From www.animalia-life.club
Boron Element What Is The Difference Between Boron 10 And Boron 11 The atomic mass of boron is 10.81 u. After neutron capture, the 10 b (n,α ) to. Boron is an element with two stable isotopes: There are 13 radioisotopes that have been. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Isotopes are chemical element atoms. What Is The Difference Between Boron 10 And Boron 11.
From www.differencebetween.com
Difference Between Boron and Borax Compare the Difference Between Similar Terms What Is The Difference Between Boron 10 And Boron 11 Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. There are 13 radioisotopes that have been. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. The atomic mass of boron is 10.81 u. Boron (5 b) naturally occurs as isotopes 10 b and. What Is The Difference Between Boron 10 And Boron 11.
From poster.4teachers.org
BORON What Is The Difference Between Boron 10 And Boron 11 Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Boron is an element with two stable isotopes: After neutron capture, the 10 b (n,α ) to. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. The. What Is The Difference Between Boron 10 And Boron 11.
From www.youtube.com
Boron has two isotopes, boron10 and boron11. Based on the average atomic mass, which isotope What Is The Difference Between Boron 10 And Boron 11 Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. After neutron capture, the 10 b (n,α ) to. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron is an element with two stable isotopes: There are 13 radioisotopes. What Is The Difference Between Boron 10 And Boron 11.
From nscl.msu.edu
Convenient location of a nearthreshold protonemitting resonance in boron11 National What Is The Difference Between Boron 10 And Boron 11 These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. After neutron capture, the 10 b (n,α ) to. There are 13 radioisotopes that have been. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Isotopes are chemical element atoms. What Is The Difference Between Boron 10 And Boron 11.
From www.differencebetween.com
Difference Between Boron and Borax Compare the Difference Between Similar Terms What Is The Difference Between Boron 10 And Boron 11 Boron is an element with two stable isotopes: After neutron capture, the 10 b (n,α ) to. The atomic mass of boron is 10.81 u. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. There are 13 radioisotopes that have been. Isotopes are chemical element atoms. What Is The Difference Between Boron 10 And Boron 11.
From www.goodscience.com.au
Atomic Number, Mass Number and Isotopes Good Science What Is The Difference Between Boron 10 And Boron 11 After neutron capture, the 10 b (n,α ) to. There are 13 radioisotopes that have been. Boron is an element with two stable isotopes: Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. These isotopes play a crucial role in understanding the mass spectrum of boron. What Is The Difference Between Boron 10 And Boron 11.
From www.slideserve.com
PPT Boron PowerPoint Presentation ID5468516 What Is The Difference Between Boron 10 And Boron 11 After neutron capture, the 10 b (n,α ) to. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. There are 13 radioisotopes that have been. The atomic mass of boron is 10.81 u. Isotopes are chemical element atoms with the same number of protons but differing. What Is The Difference Between Boron 10 And Boron 11.
From www.britannica.com
boron Properties, Uses, & Facts Britannica What Is The Difference Between Boron 10 And Boron 11 Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Boron is an element with two stable isotopes: There are 13 radioisotopes that have been. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. The atomic mass. What Is The Difference Between Boron 10 And Boron 11.
From medium.com
What is Boron? Periodic Table Elements What Is The Difference Between Boron 10 And Boron 11 Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. After neutron capture, the 10 b (n,α ) to. Boron is an element with two stable isotopes: The atomic mass of boron is 10.81 u. There are 13 radioisotopes that have been. Isotopes are chemical element atoms. What Is The Difference Between Boron 10 And Boron 11.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table What Is The Difference Between Boron 10 And Boron 11 Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. The atomic mass of boron is 10.81 u. Boron is an element with two stable isotopes: After neutron. What Is The Difference Between Boron 10 And Boron 11.
From www.youtube.com
Boron has two isotopes, B10 and B11. The average atomic mass of boron is found to be \( 10.80 What Is The Difference Between Boron 10 And Boron 11 Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. Boron is an element with two stable isotopes: Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. The atomic mass of boron is 10.81 u. There are. What Is The Difference Between Boron 10 And Boron 11.
From slideplayer.com
Physical Science Chemistry Unit 2 ppt download What Is The Difference Between Boron 10 And Boron 11 The atomic mass of boron is 10.81 u. Boron is an element with two stable isotopes: These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. After neutron capture, the 10 b (n,α ) to. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about. What Is The Difference Between Boron 10 And Boron 11.
From www.chegg.com
Solved 3. Boron has 2 naturally occurring isotopes Boron10 What Is The Difference Between Boron 10 And Boron 11 There are 13 radioisotopes that have been. After neutron capture, the 10 b (n,α ) to. The atomic mass of boron is 10.81 u. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Boron is an element with two stable isotopes: Isotopes are chemical element atoms. What Is The Difference Between Boron 10 And Boron 11.
From www.examples.com
Boron (B) Definition, Preparation, Properties, Uses, Compounds, Reactivity What Is The Difference Between Boron 10 And Boron 11 Boron is an element with two stable isotopes: Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. The atomic mass of boron is 10.81 u. There are 13 radioisotopes that have been. After neutron capture, the 10 b (n,α ) to. Isotopes are chemical element atoms. What Is The Difference Between Boron 10 And Boron 11.
From borates.today
Boron Electron Valence Borates Today What Is The Difference Between Boron 10 And Boron 11 Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. There are 13 radioisotopes that have been. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up. What Is The Difference Between Boron 10 And Boron 11.
From www.expii.com
Atomic Weight — Definition & Overview Expii What Is The Difference Between Boron 10 And Boron 11 Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. After neutron capture, the 10 b (n,α ) to. The atomic mass of boron is 10.81 u. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. There. What Is The Difference Between Boron 10 And Boron 11.
From www.differencebetween.com
Difference Between Boron and Borax Compare the Difference Between Similar Terms What Is The Difference Between Boron 10 And Boron 11 Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. There are 13 radioisotopes that have been. Boron is an element with two stable isotopes: After neutron capture, the 10 b (n,α ) to. The. What Is The Difference Between Boron 10 And Boron 11.
From nagato.cc
What Is The Difference Between Boron10 And Boron11 What Is The Difference Between Boron 10 And Boron 11 There are 13 radioisotopes that have been. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. Boron is an element with two stable isotopes: These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron (5 b) naturally occurs as isotopes 10 b and. What Is The Difference Between Boron 10 And Boron 11.
From byjus.com
boron exist as two isotopes ^10B and ^11B if atomic mass of boron in periodic table is given to What Is The Difference Between Boron 10 And Boron 11 There are 13 radioisotopes that have been. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. Boron is an element with two stable isotopes: The atomic mass of boron is 10.81 u. After neutron capture, the 10 b (n,α ) to. These isotopes play a crucial role in understanding the. What Is The Difference Between Boron 10 And Boron 11.
From pages.swcp.com
ON QUARKS, NUCLEI and BORON10 NEUTRON CAPTURE What Is The Difference Between Boron 10 And Boron 11 Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. Boron is an element with two stable isotopes: There are 13 radioisotopes that have been. The atomic mass of boron is 10.81 u. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron (5. What Is The Difference Between Boron 10 And Boron 11.
From www.slideserve.com
PPT NUCLEAR CHEMISTRY PowerPoint Presentation, free download ID3795675 What Is The Difference Between Boron 10 And Boron 11 The atomic mass of boron is 10.81 u. After neutron capture, the 10 b (n,α ) to. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Boron. What Is The Difference Between Boron 10 And Boron 11.
From www.sciencephoto.com
Boron, atomic structure Stock Image C018/3686 Science Photo Library What Is The Difference Between Boron 10 And Boron 11 These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. The atomic mass of boron is 10.81 u. After neutron capture, the 10 b (n,α ) to. Boron is an element. What Is The Difference Between Boron 10 And Boron 11.
From www.numerade.com
SOLVED1. Naturally occurring element Boron (B) with atomic number 5,is mixture of two isotopes What Is The Difference Between Boron 10 And Boron 11 Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. There are 13 radioisotopes that have been. After neutron capture, the 10 b (n,α ) to. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. These isotopes. What Is The Difference Between Boron 10 And Boron 11.
From circuitwiringchaw.z21.web.core.windows.net
Boron Lewis Diagram What Is The Difference Between Boron 10 And Boron 11 After neutron capture, the 10 b (n,α ) to. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Boron is an element with two stable isotopes: These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Isotopes are chemical element. What Is The Difference Between Boron 10 And Boron 11.
From www.animalia-life.club
Physical Properties Of Boron What Is The Difference Between Boron 10 And Boron 11 There are 13 radioisotopes that have been. The atomic mass of boron is 10.81 u. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. Boron is an element with two stable isotopes: These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron (5. What Is The Difference Between Boron 10 And Boron 11.
From material-properties.org
Boron Periodic Table and Atomic Properties What Is The Difference Between Boron 10 And Boron 11 Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. There are 13 radioisotopes that have been. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Isotopes are chemical element atoms with the same number of protons but differing numbers. What Is The Difference Between Boron 10 And Boron 11.
From www.americanelements.com
Boron10 Isotope AMERICAN ELEMENTS What Is The Difference Between Boron 10 And Boron 11 Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. The atomic mass of boron is 10.81 u. After neutron capture, the 10 b (n,α ) to. Boron. What Is The Difference Between Boron 10 And Boron 11.
From www.nuclear-power.com
Boron Atomic Number Atomic Mass Density of Boron What Is The Difference Between Boron 10 And Boron 11 After neutron capture, the 10 b (n,α ) to. The atomic mass of boron is 10.81 u. There are 13 radioisotopes that have been. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Isotopes are chemical element atoms with the same number of protons but differing. What Is The Difference Between Boron 10 And Boron 11.
From www.slideserve.com
PPT Boron PowerPoint Presentation ID5468516 What Is The Difference Between Boron 10 And Boron 11 Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. Boron is an element with two stable isotopes: The atomic mass of boron is 10.81 u. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron (5 b) naturally occurs as isotopes 10 b. What Is The Difference Between Boron 10 And Boron 11.
From www.numerade.com
SOLVED The two naturally occurring isotopes of boron, boron10 and boron11, have masses of10 What Is The Difference Between Boron 10 And Boron 11 These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron is an element with two stable isotopes: After neutron capture, the 10 b (n,α ) to. There are 13 radioisotopes that have been. Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. The. What Is The Difference Between Boron 10 And Boron 11.
From tagvault.org
Borax vs Boron (Explained) What Is The Difference Between Boron 10 And Boron 11 Boron is an element with two stable isotopes: After neutron capture, the 10 b (n,α ) to. There are 13 radioisotopes that have been. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. Isotopes are chemical element atoms with the same number of protons but differing. What Is The Difference Between Boron 10 And Boron 11.
From www.alamy.com
Boron Atomic Structure High Resolution Stock Photography and Images Alamy What Is The Difference Between Boron 10 And Boron 11 These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. After neutron capture, the 10 b (n,α ) to. There are 13 radioisotopes that have been. Isotopes are chemical element atoms. What Is The Difference Between Boron 10 And Boron 11.
From utedzz.blogspot.com
Periodic Table Boron Atom Periodic Table Timeline What Is The Difference Between Boron 10 And Boron 11 These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron (5 b) naturally occurs as isotopes 10 b and 11 b, the latter of which makes up about 80% of natural boron. The atomic mass of boron is 10.81 u. Isotopes are chemical element atoms with the same number of protons but differing. What Is The Difference Between Boron 10 And Boron 11.
From www.numerade.com
The element boron has two stable isotopes boron10 (10.0129 u) and boron11 (11.0093 u). The What Is The Difference Between Boron 10 And Boron 11 There are 13 radioisotopes that have been. After neutron capture, the 10 b (n,α ) to. These isotopes play a crucial role in understanding the mass spectrum of boron and its applications. Boron is an element with two stable isotopes: Isotopes are chemical element atoms with the same number of protons but differing numbers of neutrons in their nuclei. Boron. What Is The Difference Between Boron 10 And Boron 11.