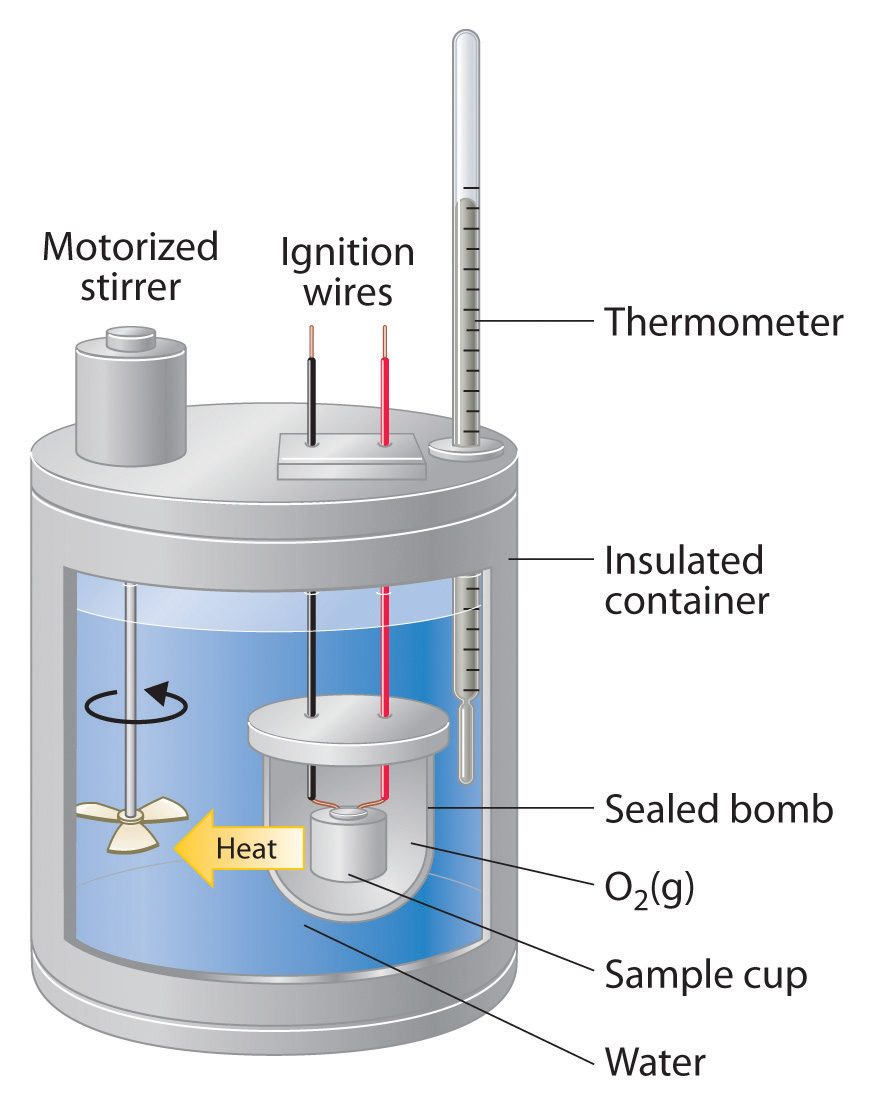
Combustion Calorimeter Heat Capacity . The heat capacity of the bomb calorimeter is 2.36 kj/ °c. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. 1.150 g of sucrose goes through combustion in a bomb calorimeter. Calculate the amount of heat released during the. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. Because the combustion of butane. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the.
from 2012books.lardbucket.org
Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Calculate the amount of heat released during the. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. 1.150 g of sucrose goes through combustion in a bomb calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. Because the combustion of butane.
Calorimetry
Combustion Calorimeter Heat Capacity Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. 1.150 g of sucrose goes through combustion in a bomb calorimeter. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. Because the combustion of butane. Calculate the amount of heat released during the. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat capacity of the bomb calorimeter is 2.36 kj/ °c.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Combustion Calorimeter Heat Capacity If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. Calculate the amount of heat released during. Combustion Calorimeter Heat Capacity.
From 2012books.lardbucket.org
Calorimetry Combustion Calorimeter Heat Capacity The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. 1.150 g of sucrose goes through combustion in a bomb calorimeter. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the.. Combustion Calorimeter Heat Capacity.
From users.highland.edu
Calorimetry Combustion Calorimeter Heat Capacity If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. Because the combustion of butane. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Calculate the amount of heat. Combustion Calorimeter Heat Capacity.
From www.slideserve.com
PPT Unit 4 Thermochemistry & Thermodynamics PowerPoint Presentation Combustion Calorimeter Heat Capacity 1.150 g of sucrose goes through combustion in a bomb calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate the amount of heat released during the. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. If $\pu{1.25 g}$ of glucose. Combustion Calorimeter Heat Capacity.
From www.animalia-life.club
Calorimeter Diagram Combustion Calorimeter Heat Capacity To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). The combustion of. Combustion Calorimeter Heat Capacity.
From www.thoughtco.com
Calorimeter Definition in Chemistry Combustion Calorimeter Heat Capacity If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. 1.150 g of sucrose goes through combustion in a bomb calorimeter. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g). Combustion Calorimeter Heat Capacity.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Combustion Calorimeter Heat Capacity Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Calculate the amount of heat released during the. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. If the temperature rose from 23.42 °c to 27.64 °c and the. Combustion Calorimeter Heat Capacity.
From www.chegg.com
Solved Combustion (bomb) calorimeter. Calculate the heat Combustion Calorimeter Heat Capacity To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. Calculate the amount of heat released during the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. 2c 4 h 10 (g) + 13o 2 (g) →. Combustion Calorimeter Heat Capacity.
From ar.inspiredpencil.com
Heat Of Combustion Lab Combustion Calorimeter Heat Capacity A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. Then use equation 5.6.21 to. Combustion Calorimeter Heat Capacity.
From chemistryvce.weebly.com
Measuring energy VCE Chemistry Combustion Calorimeter Heat Capacity Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. Calculate the amount. Combustion Calorimeter Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Combustion Calorimeter Heat Capacity A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. 1.150 g of sucrose goes through combustion in a bomb calorimeter. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. 2c 4 h 10 (g). Combustion Calorimeter Heat Capacity.
From www.hanlin.com
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Combustion Calorimeter Heat Capacity For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Because the combustion of butane. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calculate the amount of heat released during the. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) +. Combustion Calorimeter Heat Capacity.
From www.chegg.com
Solved Calculate the heat capacity of a calorimeter if the Combustion Calorimeter Heat Capacity Because the combustion of butane. 1.150 g of sucrose goes through combustion in a bomb calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity. Combustion Calorimeter Heat Capacity.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Combustion Calorimeter Heat Capacity A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. Then. Combustion Calorimeter Heat Capacity.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Combustion Calorimeter Heat Capacity 1.150 g of sucrose goes through combustion in a bomb calorimeter. Calculate the amount of heat released during the. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. The combustion of 1 mole of glucose $\ce{c6h12o6}$. Combustion Calorimeter Heat Capacity.
From www.answersarena.com
[Solved] Combustion (bomb) calorimeter. The heat capacity Combustion Calorimeter Heat Capacity If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. 1.150 g of sucrose goes through combustion in a bomb calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Combustion Calorimeter Heat Capacity.
From joiwmxbys.blob.core.windows.net
Calorimetry With Two Solutions at Angelica Kirkland blog Combustion Calorimeter Heat Capacity If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. 1.150 g of sucrose goes through combustion in a bomb calorimeter. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat. Combustion Calorimeter Heat Capacity.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Combustion Calorimeter Heat Capacity If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. Because the combustion of butane. The. Combustion Calorimeter Heat Capacity.
From slidetodoc.com
Calorimetry Heat of combustion Heat capacity Solution calorimetry Combustion Calorimeter Heat Capacity The heat capacity of the bomb calorimeter is 2.36 kj/ °c. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. 1.150 g of sucrose goes through combustion in a bomb calorimeter. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). If $\pu{1.25. Combustion Calorimeter Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Combustion Calorimeter Heat Capacity For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Because the combustion of butane. To find the heat capacity of the calorimeter, we need to. Combustion Calorimeter Heat Capacity.
From socratic.org
When 0.602 g of biphenyl (C12H10) undergoes combustion in a bomb Combustion Calorimeter Heat Capacity A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. Calculate the amount of heat released during the. 1.150 g of sucrose goes. Combustion Calorimeter Heat Capacity.
From www.science-revision.co.uk
Calorimetry Combustion Calorimeter Heat Capacity For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. If the temperature rose from 23.42 °c to 27.64 °c. Combustion Calorimeter Heat Capacity.
From www.numerade.com
SOLVED Use the References to access important values if needed for Combustion Calorimeter Heat Capacity Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). Because the combustion of butane. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. The heat capacity of. Combustion Calorimeter Heat Capacity.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Combustion Calorimeter Heat Capacity To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. Calculate the amount of heat released during. Combustion Calorimeter Heat Capacity.
From www.toppr.com
Calculate the heat of combustion of methane at constant volume. The Combustion Calorimeter Heat Capacity 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for. Combustion Calorimeter Heat Capacity.
From www.numerade.com
SOLVED Abomb calorimeter; Or a constant volume calorimeter; is a Combustion Calorimeter Heat Capacity 1.150 g of sucrose goes through combustion in a bomb calorimeter. Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. Calculate the amount of heat released during the. For example, when an exothermic reaction occurs. Combustion Calorimeter Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Combustion Calorimeter Heat Capacity If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of water and the. To find the heat capacity of the calorimeter, we need to combust. Combustion Calorimeter Heat Capacity.
From www.chegg.com
Solved Calculate the heat capacity of a calorimeter if the Combustion Calorimeter Heat Capacity To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. Calculate the amount of heat released during the. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2. Combustion Calorimeter Heat Capacity.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Combustion Calorimeter Heat Capacity A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. 1.150 g of sucrose goes through combustion in a bomb calorimeter. To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. The heat capacity of the bomb calorimeter. Combustion Calorimeter Heat Capacity.
From www.numerade.com
SOLVED A bomb calorimeter or a constant volume calorimeler; is a Combustion Calorimeter Heat Capacity If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. Because the combustion of butane. Calculate the amount of heat released during the. 1.150 g of sucrose goes through combustion in a bomb calorimeter. For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. To find the heat capacity. Combustion Calorimeter Heat Capacity.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Combustion Calorimeter Heat Capacity Then use equation 5.6.21 to determine the heat capacity of the calorimeter (\(c_{bomb}\)) from \(q_{comb}\) and \(δt\). For example, when an exothermic reaction occurs in solution in a calorimeter, the heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3. Combustion Calorimeter Heat Capacity.
From www.chegg.com
Solved Combustion (bomb) calorimeter. In an experiment, a Combustion Calorimeter Heat Capacity To find the heat capacity of the calorimeter, we need to combust something that we know the exact amount of heat for them. 1.150 g of sucrose goes through combustion in a bomb calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Then use equation 5.6.21 to determine the. Combustion Calorimeter Heat Capacity.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Combustion Calorimeter Heat Capacity 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. To find the heat capacity of the calorimeter, we need to. Combustion Calorimeter Heat Capacity.
From courses.lumenlearning.com
Calorimetry General Chemistry Combustion Calorimeter Heat Capacity 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. If the temperature rose from 23.42 °c to 27.64 °c and the heat capacity of the calorimeter. The heat capacity of the bomb calorimeter is 2.36 kj/ °c. Because the combustion of butane. For example, when an exothermic reaction occurs in. Combustion Calorimeter Heat Capacity.
From www.numerade.com
SOLVED A bomb calorimeter can be used to measure the enthalpy of Combustion Calorimeter Heat Capacity 1.150 g of sucrose goes through combustion in a bomb calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. 2c 4 h 10 (g) + 13o 2 (g) → 8co 2 (g) + 10h 2 o (g) solution. Then use equation 5.6.21 to determine the heat capacity of the. Combustion Calorimeter Heat Capacity.