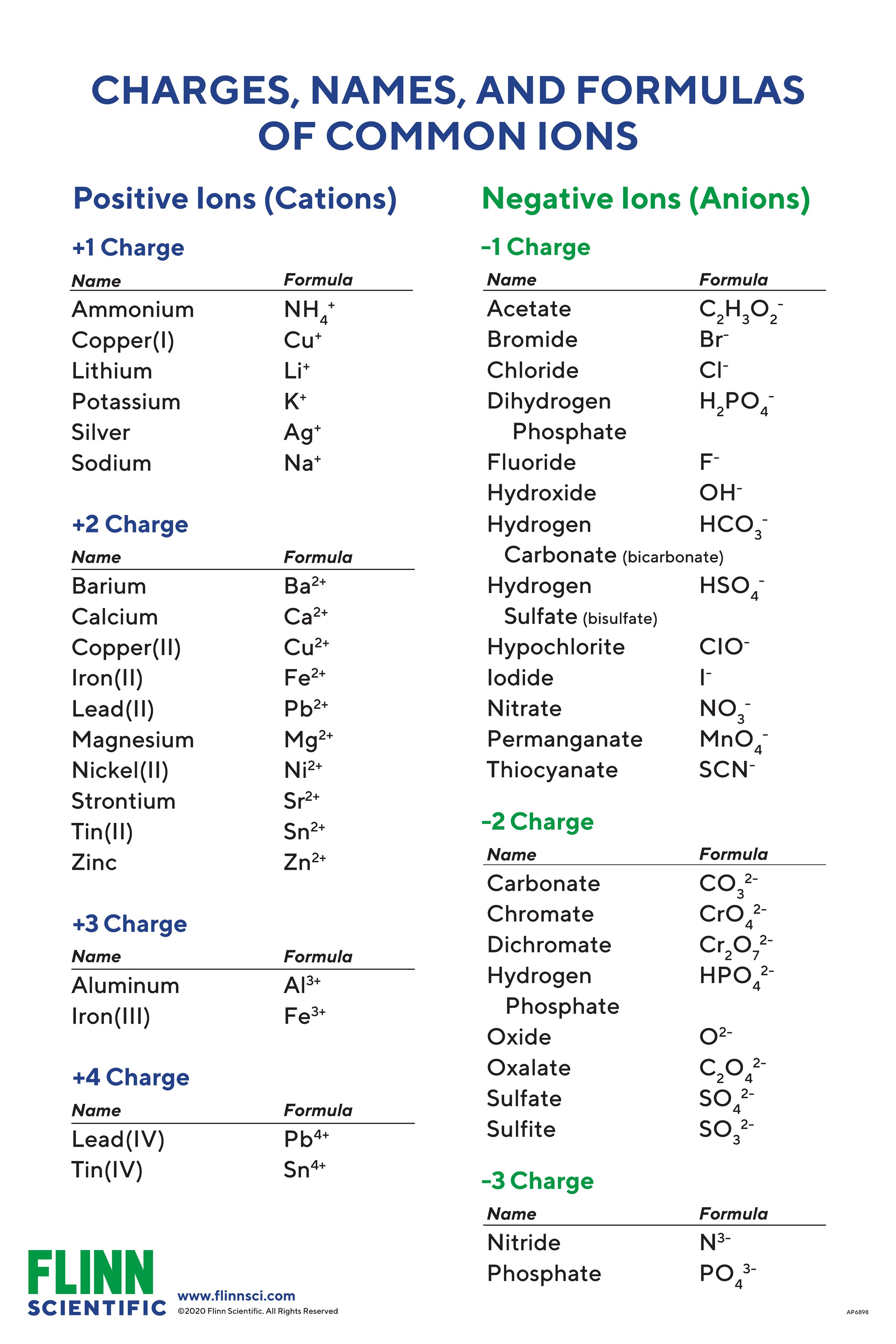
Copper Charge Ion . 93 rows ionic charge: Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). Example of some compounds that have multiple oxidation states. 93 rows this table shows the most common charges for atoms of the chemical elements. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. Copper (i) and copper (ii). Roman numeral notation indicates charge of ion when element commonly forms. This electric charge generated on. Copper (i) ions have a 1^+ charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This happens when copper atoms lose one electron.
from
When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows this table shows the most common charges for atoms of the chemical elements. This happens when copper atoms lose one electron. 93 rows ionic charge: Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. Roman numeral notation indicates charge of ion when element commonly forms. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). Copper (i) ions have a 1^+ charge. Copper (i) and copper (ii).
Copper Charge Ion Copper (i) and copper (ii). This happens when copper atoms lose one electron. 93 rows ionic charge: Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. This electric charge generated on. Copper (i) ions have a 1^+ charge. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). 93 rows this table shows the most common charges for atoms of the chemical elements. Example of some compounds that have multiple oxidation states. Roman numeral notation indicates charge of ion when element commonly forms. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Copper (i) and copper (ii).
From
Copper Charge Ion 93 rows this table shows the most common charges for atoms of the chemical elements. Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. Copper (i) ions have a 1^+ charge. Example of some compounds that have multiple oxidation states. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This happens. Copper Charge Ion.
From www.chegg.com
Solved Model 1 Ion Charges for Selected Elements H' 1 Li* 2 Copper Charge Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on. Copper (i) and copper (ii). Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. 93 rows ionic charge: Copper (i) ions have a 1^+ charge.. Copper Charge Ion.
From foptbest.weebly.com
Periodic table of elements with ion charges foptbest Copper Charge Ion Example of some compounds that have multiple oxidation states. Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. This electric charge generated on. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii).. Copper Charge Ion.
From
Copper Charge Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Copper (i) ions have a 1^+ charge. 93 rows this table shows the most common charges for atoms of the chemical elements. This electric charge generated on. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water. Copper Charge Ion.
From
Copper Charge Ion Example of some compounds that have multiple oxidation states. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on. Hydroxide ions (from, say, sodium hydroxide. Copper Charge Ion.
From pubs.rsc.org
Cu 2+ selective chelators relieve copperinduced oxidative stress in Copper Charge Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Copper (i) and copper (ii). This happens when copper atoms lose one electron. Example of some compounds that have multiple oxidation states. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. 93 rows. Copper Charge Ion.
From www.youtube.com
Cation Test Copper(II) Ions YouTube Copper Charge Ion Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Copper (i) ions have a 1^+ charge. 93 rows this table shows the most common charges for atoms of the chemical elements. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. This electric charge. Copper Charge Ion.
From www.sciencephoto.com
Copper hydroxide precipitate Stock Image A500/0411 Science Photo Copper Charge Ion 93 rows this table shows the most common charges for atoms of the chemical elements. Example of some compounds that have multiple oxidation states. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. This happens when copper atoms lose one electron. Copper (i) ions have a 1^+ charge. Copper(ii) cu2+. Copper Charge Ion.
From
Copper Charge Ion Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). When the atom loses or gains one or more electrons, the electric charge is generated (and an. Copper Charge Ion.
From
Copper Charge Ion Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). 93 rows ionic charge: Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Copper(ii). Copper Charge Ion.
From
Copper Charge Ion Copper (i) and copper (ii). Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Roman numeral notation indicates charge of ion when element commonly forms. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Copper (i). Copper Charge Ion.
From
Copper Charge Ion Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. Copper (i) ions have a 1^+ charge. This happens when copper atoms lose one electron. Example of some compounds that have multiple oxidation states. 93 rows ionic charge: This electric charge generated on. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed).. Copper Charge Ion.
From
Copper Charge Ion Example of some compounds that have multiple oxidation states. Copper (i) and copper (ii). Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. Roman numeral notation indicates charge of ion when element commonly forms. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Copper (i) ions have. Copper Charge Ion.
From www.youtube.com
How to find Protons & Electrons for Cu+ and Cu2+ (Copper II and III Copper Charge Ion Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. 93 rows this table shows the most common charges for atoms of the chemical elements. Roman numeral notation indicates charge of ion when element commonly forms. Copper (i) and copper (ii). Example of some compounds that have multiple oxidation states. Learn. Copper Charge Ion.
From chemistry.com.pk
Colours of Transition Metal Ions in Aqueous Solution [Infographic Copper Charge Ion 93 rows ionic charge: Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). Copper (i) ions have a 1^+ charge. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. This electric charge generated on. Roman numeral notation indicates charge. Copper Charge Ion.
From
Copper Charge Ion Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. This happens when copper atoms lose one electron. Roman numeral notation indicates charge of ion when element commonly forms. Copper (i) and copper (ii). Copper(ii). Copper Charge Ion.
From
Copper Charge Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. This happens when copper atoms lose one electron. Learn how to name and write the formula of ions with variable charges,. Copper Charge Ion.
From
Copper Charge Ion Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). This electric charge generated on. When the atom loses or gains one or more electrons, the electric charge is generated (and. Copper Charge Ion.
From
Copper Charge Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Copper (i) and copper (ii). Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Example of some compounds that have multiple oxidation states. Note mercury (1) is not a monoatomic. Copper Charge Ion.
From www.nagwa.com
Question Video Atomic Structure of a Copper Ion Nagwa Copper Charge Ion Example of some compounds that have multiple oxidation states. Roman numeral notation indicates charge of ion when element commonly forms. Copper (i) and copper (ii). This electric charge generated on. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. 93 rows this table shows the most common charges for atoms of the chemical. Copper Charge Ion.
From
Copper Charge Ion Copper (i) ions have a 1^+ charge. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). 93 rows this table shows the most common charges for atoms of the. Copper Charge Ion.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Copper Have? Copper Charge Ion Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. 93 rows this table shows the most common charges for atoms of the chemical elements. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Learn how. Copper Charge Ion.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Charge Ion This happens when copper atoms lose one electron. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Roman numeral notation indicates charge of ion when element commonly forms. Copper(ii) cu2+. Copper Charge Ion.
From www.es.crujera.com
Part 1 Background Alfonso Crujera Copper Charge Ion 93 rows this table shows the most common charges for atoms of the chemical elements. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Note mercury (1) is not a. Copper Charge Ion.
From
Copper Charge Ion When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion.. Copper Charge Ion.
From
Copper Charge Ion This electric charge generated on. Example of some compounds that have multiple oxidation states. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). 93 rows ionic charge: Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. When the atom. Copper Charge Ion.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Charge Ion Copper (i) and copper (ii). Example of some compounds that have multiple oxidation states. This happens when copper atoms lose one electron. 93 rows ionic charge: Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). When the atom loses or gains one or more electrons, the electric charge is. Copper Charge Ion.
From
Copper Charge Ion 93 rows this table shows the most common charges for atoms of the chemical elements. Roman numeral notation indicates charge of ion when element commonly forms. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Learn how to name and write the formula of ions with variable charges, such as copper (i) and. Copper Charge Ion.
From
Copper Charge Ion Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Example of some compounds that have multiple oxidation states. Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. Roman numeral notation indicates charge of ion when element commonly forms. Learn how to name and write the formula of ions with variable charges, such as copper (i). Copper Charge Ion.
From
Copper Charge Ion This electric charge generated on. Copper (i) and copper (ii). Example of some compounds that have multiple oxidation states. Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. This happens when copper atoms lose one electron. 93 rows ionic charge: Roman numeral notation indicates charge of. Copper Charge Ion.
From 9to5science.com
[Solved] Copper metal with silver ion 9to5Science Copper Charge Ion Roman numeral notation indicates charge of ion when element commonly forms. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. Example of some compounds that have multiple oxidation states. This electric charge generated. Copper Charge Ion.
From saylordotorg.github.io
Ionic Bonding and Simple Ionic Compounds Copper Charge Ion 93 rows this table shows the most common charges for atoms of the chemical elements. Learn how to name and write the formula of ions with variable charges, such as copper (i) and copper (ii). Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of. This electric charge generated on. Example of some compounds. Copper Charge Ion.
From
Copper Charge Ion Copper (i) ions have a 1^+ charge. Copper(ii) cu2+ iron(ii) fe2+ lead(ii) pb2+ magnesium. This electric charge generated on. Example of some compounds that have multiple oxidation states. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Copper (i) and copper (ii). This happens when. Copper Charge Ion.
From
Copper Charge Ion Example of some compounds that have multiple oxidation states. Roman numeral notation indicates charge of ion when element commonly forms. 93 rows this table shows the most common charges for atoms of the chemical elements. Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. This electric charge generated on. Copper. Copper Charge Ion.
From general.chemistrysteps.com
Naming Monatomic and Polyatomic Ions Chemistry Steps Copper Charge Ion Example of some compounds that have multiple oxidation states. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Copper (i) and copper (ii). Hydroxide ions (from, say, sodium hydroxide solution) remove hydrogen ions from the water ligands attached to the copper ion. Note mercury (1) is not a monoatomic. Copper Charge Ion.