Is Bromine Electron Withdrawing . The substituents on the ring are groups that donate electrons. The substituents on the ring are groups that withdraw electrons. For example, take the bromination reaction of nitrobenzene, shown in the next figure. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. The yields aren’t great, but there you go. Substituents with several bonds to electronegative atoms (e.g. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen.
from www.numerade.com
Substituents with several bonds to electronegative atoms (e.g. The yields aren’t great, but there you go. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. For example, take the bromination reaction of nitrobenzene, shown in the next figure. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. The substituents on the ring are groups that withdraw electrons. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. The substituents on the ring are groups that donate electrons.
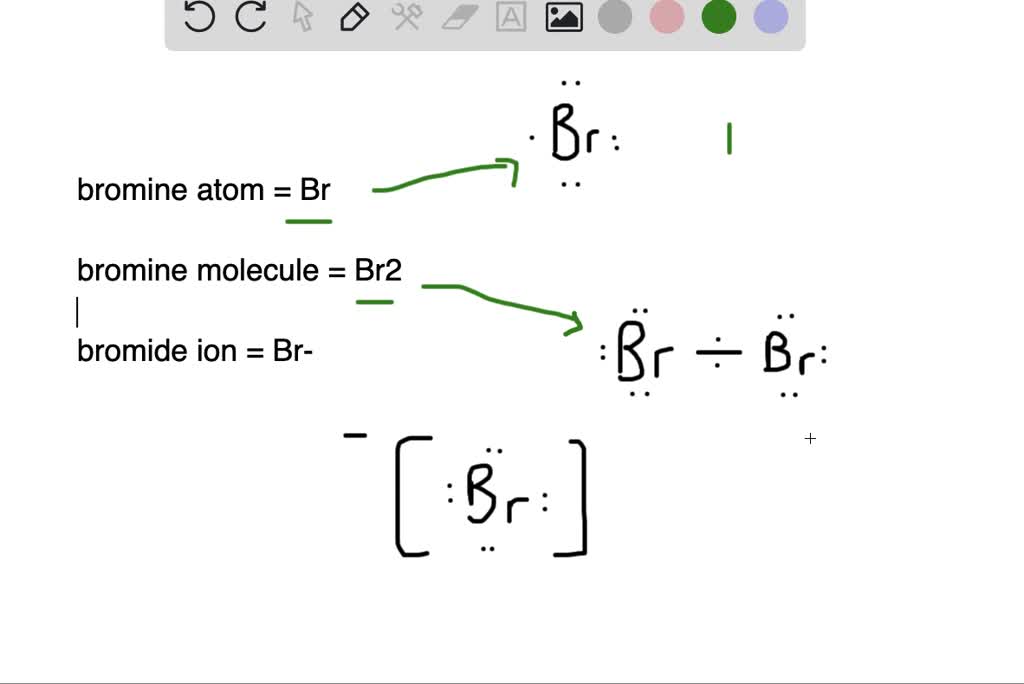
What is the difference between (a) a bromine atom, (b) a bromine
Is Bromine Electron Withdrawing The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. The yields aren’t great, but there you go. Substituents with several bonds to electronegative atoms (e.g. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. The substituents on the ring are groups that withdraw electrons. For example, take the bromination reaction of nitrobenzene, shown in the next figure. The substituents on the ring are groups that donate electrons. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep Is Bromine Electron Withdrawing The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Substituents with several bonds to electronegative atoms (e.g. For example, take the bromination reaction of nitrobenzene, shown in the next figure. The substituents on the ring are groups that donate electrons. The yields aren’t great, but there you go.. Is Bromine Electron Withdrawing.
From www.schoolmykids.com
Br Bromine Element Information Facts, Properties, Trends, Uses and Is Bromine Electron Withdrawing The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. The yields aren’t great, but there you go. The substituents on the ring are groups that withdraw electrons. The substituents on the ring are groups that donate electrons. Substituents with several bonds to electronegative atoms (e.g. Knowing what we. Is Bromine Electron Withdrawing.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Is Bromine Electron Withdrawing For example, take the bromination reaction of nitrobenzene, shown in the next figure. Substituents with several bonds to electronegative atoms (e.g. The substituents on the ring are groups that withdraw electrons. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair. Is Bromine Electron Withdrawing.
From app.emaze.com
Bromine ) on emaze Is Bromine Electron Withdrawing Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. For example, take the bromination reaction of nitrobenzene, shown in the next figure. The substituents on the ring are groups that withdraw electrons. Since the rate is so. Is Bromine Electron Withdrawing.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Is Bromine Electron Withdrawing Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. The substituents on the ring are groups. Is Bromine Electron Withdrawing.
From www.youtube.com
Br electronic configurationHow to write electronic configuration of Is Bromine Electron Withdrawing For example, take the bromination reaction of nitrobenzene, shown in the next figure. Substituents with several bonds to electronegative atoms (e.g. The yields aren’t great, but there you go. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. The substituents on the ring are groups that donate electrons.. Is Bromine Electron Withdrawing.
From www.animalia-life.club
Electron Configuration For Bromine Is Bromine Electron Withdrawing Substituents with several bonds to electronegative atoms (e.g. For example, take the bromination reaction of nitrobenzene, shown in the next figure. The substituents on the ring are groups that donate electrons. The substituents on the ring are groups that withdraw electrons. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that. Is Bromine Electron Withdrawing.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Is Bromine Electron Withdrawing The substituents on the ring are groups that withdraw electrons. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair. Is Bromine Electron Withdrawing.
From www.youtube.com
What is Electron Withdrawing Groups ? Inductive effect & Acidity Is Bromine Electron Withdrawing The yields aren’t great, but there you go. The substituents on the ring are groups that withdraw electrons. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. For. Is Bromine Electron Withdrawing.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Is Bromine Electron Withdrawing The substituents on the ring are groups that donate electrons. The substituents on the ring are groups that withdraw electrons. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. The yields aren’t great, but there you go. Knowing what we now know about halogens, what predictions would you make. Is Bromine Electron Withdrawing.
From www.chegg.com
Solved a) Bromine is an electron withdrawing group, however, Is Bromine Electron Withdrawing The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. For example, take the bromination reaction of nitrobenzene, shown in the next figure. The substituents on the ring are groups that withdraw electrons. The yields aren’t great, but there you go. Since the rate is so sensitive to whether. Is Bromine Electron Withdrawing.
From www.animalia-life.club
Electron Configuration For Bromine Is Bromine Electron Withdrawing The substituents on the ring are groups that donate electrons. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. Substituents with several bonds to electronegative atoms (e.g. The withdrawal or donation of electrons by a substituent group. Is Bromine Electron Withdrawing.
From www.animalia-life.club
Electron Configuration For Bromine Is Bromine Electron Withdrawing The substituents on the ring are groups that withdraw electrons. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair. Is Bromine Electron Withdrawing.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Is Bromine Electron Withdrawing The yields aren’t great, but there you go. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. For example, take the bromination reaction of nitrobenzene, shown in the next figure. The withdrawal or donation of electrons by. Is Bromine Electron Withdrawing.
From www.animalia-life.club
Electron Configuration For Bromine Is Bromine Electron Withdrawing Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. The substituents on the ring are groups that withdraw electrons. The substituents on the ring are groups that donate electrons. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is. Is Bromine Electron Withdrawing.
From lambdageeks.com
Bromine Electron Configuration 7 Easy Steps on How to Write Is Bromine Electron Withdrawing The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Substituents with several bonds to electronegative atoms (e.g. The substituents on the ring are groups that donate electrons. The yields aren’t great, but there you go. Knowing what we now know about halogens, what predictions would you make for. Is Bromine Electron Withdrawing.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Is Bromine Electron Withdrawing For example, take the bromination reaction of nitrobenzene, shown in the next figure. The substituents on the ring are groups that withdraw electrons. The substituents on the ring are groups that donate electrons. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a. Is Bromine Electron Withdrawing.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Is Bromine Electron Withdrawing The yields aren’t great, but there you go. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Substituents with several bonds to electronegative atoms (e.g. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. The substituents on. Is Bromine Electron Withdrawing.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Is Bromine Electron Withdrawing The substituents on the ring are groups that donate electrons. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. The yields aren’t great, but there you go. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. Substituents. Is Bromine Electron Withdrawing.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Is Bromine Electron Withdrawing For example, take the bromination reaction of nitrobenzene, shown in the next figure. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. Since the rate is so sensitive to whether the group is electron donating or electron. Is Bromine Electron Withdrawing.
From www.animalia-life.club
Electron Configuration For Bromine Is Bromine Electron Withdrawing Substituents with several bonds to electronegative atoms (e.g. The yields aren’t great, but there you go. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. The substituents on. Is Bromine Electron Withdrawing.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Is Bromine Electron Withdrawing Substituents with several bonds to electronegative atoms (e.g. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. The yields aren’t great, but there you go. The withdrawal or donation of electrons by a substituent group is controlled. Is Bromine Electron Withdrawing.
From ar.inspiredpencil.com
Electron Configuration For Bromine Is Bromine Electron Withdrawing For example, take the bromination reaction of nitrobenzene, shown in the next figure. The substituents on the ring are groups that donate electrons. The substituents on the ring are groups that withdraw electrons. The yields aren’t great, but there you go. Substituents with several bonds to electronegative atoms (e.g. The withdrawal or donation of electrons by a substituent group is. Is Bromine Electron Withdrawing.
From forums.studentdoctor.net
Resonance Electron Withdrawing or Donating Student Doctor Network Is Bromine Electron Withdrawing For example, take the bromination reaction of nitrobenzene, shown in the next figure. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. The yields aren’t great, but there you go. The withdrawal or donation of electrons by. Is Bromine Electron Withdrawing.
From www.youtube.com
Super Trick Electronic configuration of Bromine Bromine electronic Is Bromine Electron Withdrawing Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. For example, take the bromination reaction of nitrobenzene,. Is Bromine Electron Withdrawing.
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide Is Bromine Electron Withdrawing For example, take the bromination reaction of nitrobenzene, shown in the next figure. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a. Is Bromine Electron Withdrawing.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Is Bromine Electron Withdrawing The substituents on the ring are groups that donate electrons. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. The yields aren’t great, but there you go. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron. Is Bromine Electron Withdrawing.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Is Bromine Electron Withdrawing Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. The substituents on the ring are groups that withdraw electrons. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Knowing what we now know about halogens, what predictions. Is Bromine Electron Withdrawing.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Is Bromine Electron Withdrawing Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. Substituents with several bonds to electronegative atoms (e.g.. Is Bromine Electron Withdrawing.
From www.clutchprep.com
Electron Withdrawing Groups Organic Chemistry Video Clutch Prep Is Bromine Electron Withdrawing The substituents on the ring are groups that withdraw electrons. The yields aren’t great, but there you go. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. For example, take the bromination reaction of nitrobenzene, shown in the next figure. The substituents on the ring are groups that. Is Bromine Electron Withdrawing.
From material-properties.org
Bromine Periodic Table and Atomic Properties Is Bromine Electron Withdrawing Substituents with several bonds to electronegative atoms (e.g. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the. Is Bromine Electron Withdrawing.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine Is Bromine Electron Withdrawing The substituents on the ring are groups that withdraw electrons. The yields aren’t great, but there you go. For example, take the bromination reaction of nitrobenzene, shown in the next figure. Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. Knowing what we now know about halogens, what predictions. Is Bromine Electron Withdrawing.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Is Bromine Electron Withdrawing Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. Substituents with several bonds to electronegative atoms (e.g. The substituents on the ring are groups that withdraw electrons. The yields aren’t great, but there you go. For example, take the bromination reaction of nitrobenzene, shown in the next figure. The. Is Bromine Electron Withdrawing.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Is Bromine Electron Withdrawing Since the rate is so sensitive to whether the group is electron donating or electron withdrawing (“electronic effects”, as organic. The withdrawal or donation of electrons by a substituent group is controlled by an interplay of inductive effects and resonance effects. Substituents with several bonds to electronegative atoms (e.g. The substituents on the ring are groups that donate electrons. The. Is Bromine Electron Withdrawing.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Is Bromine Electron Withdrawing For example, take the bromination reaction of nitrobenzene, shown in the next figure. Knowing what we now know about halogens, what predictions would you make for the nitroso group, a group that is somewhat electron withdrawing, but also bears a lone pair on the nitrogen. Substituents with several bonds to electronegative atoms (e.g. Since the rate is so sensitive to. Is Bromine Electron Withdrawing.