What Is Electrode Positive . the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. the pt electrode in the permanganate solution is the cathode; in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. the battery’s negative electrode is the anode. Its positive electrode is the cathode. The cathode (electrode in beaker that. The one in the tin solution is the anode. Since electrons are negatively charged, they naturally flow from the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode.
from cemcnutz.blob.core.windows.net
the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. The cathode (electrode in beaker that. Since electrons are negatively charged, they naturally flow from the. the battery’s negative electrode is the anode. the pt electrode in the permanganate solution is the cathode; in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Its positive electrode is the cathode. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. The one in the tin solution is the anode.
What Are Electrodes Class 8 at Edith re blog
What Is Electrode Positive in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. the pt electrode in the permanganate solution is the cathode; the battery’s negative electrode is the anode. The cathode (electrode in beaker that. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Its positive electrode is the cathode. Since electrons are negatively charged, they naturally flow from the. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. The one in the tin solution is the anode.
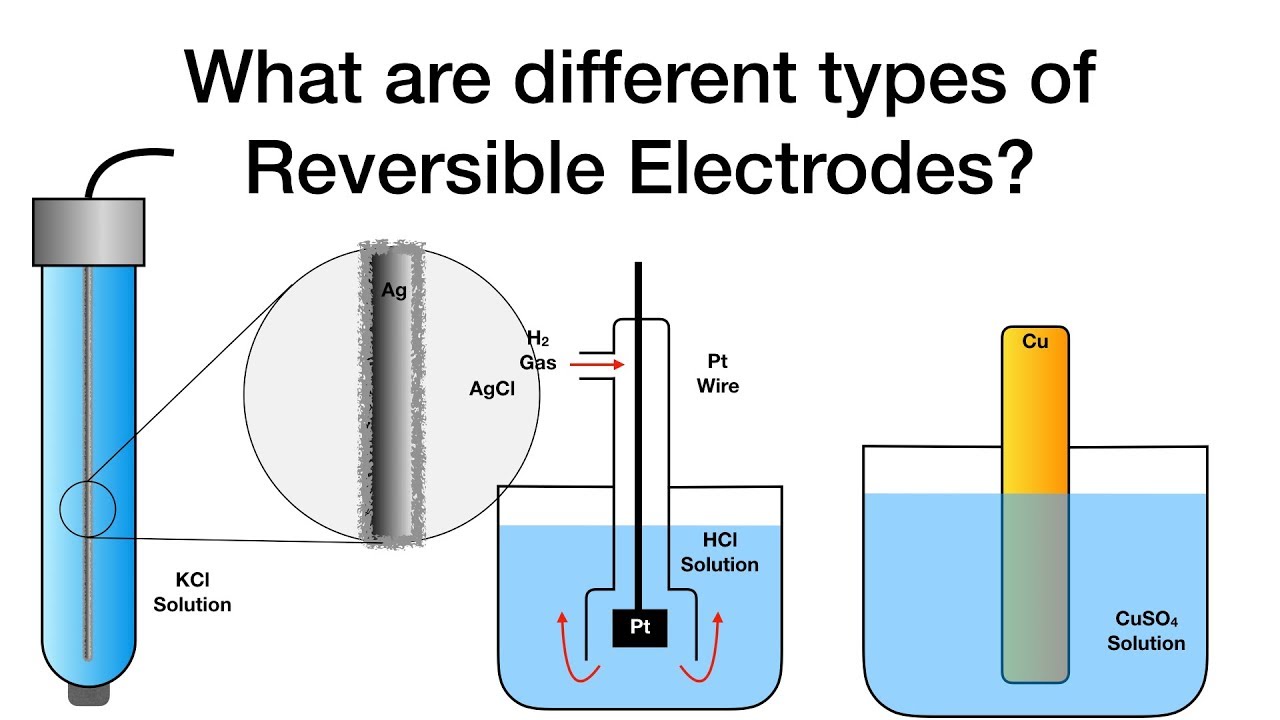
From study.com
Electrodes Definition & Types Video & Lesson Transcript What Is Electrode Positive The one in the tin solution is the anode. The cathode (electrode in beaker that. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Its positive electrode is the cathode.. What Is Electrode Positive.
From www.quinden.co
electrode positif anode et cathode définition Schleun What Is Electrode Positive if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. the electrode attached to the positive terminal of a battery is. What Is Electrode Positive.
From www.freepik.com
Premium Photo Ions movement to negative electrode and positive electrode What Is Electrode Positive in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Its positive electrode is the cathode. the battery’s negative electrode is the anode. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the. What Is Electrode Positive.
From www.researchgate.net
Voltammograms at 1mVs−1 of negative and positive electrodes vs. NHE for What Is Electrode Positive in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. Its positive electrode is the cathode. the pt. What Is Electrode Positive.
From weldingclarity.com
Is Stick Welding Electrode Positive Or Negative? Detailed What Is Electrode Positive Since electrons are negatively charged, they naturally flow from the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The one in the tin solution is the anode. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. The cathode. What Is Electrode Positive.
From byjus.com
In electrolysis what are the positive and negative electrodes known as?? What Is Electrode Positive if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The one in the tin solution is the anode. Since electrons are. What Is Electrode Positive.
From thoracickey.com
ECG Thoracic Key What Is Electrode Positive The one in the tin solution is the anode. Its positive electrode is the cathode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. the pt electrode in the permanganate solution is the cathode; if electrons flow from the left electrode to the right electrode (as depicted in the. What Is Electrode Positive.
From www.researchgate.net
Negative and positive electrode materials for LIBs. Blue outlined What Is Electrode Positive Its positive electrode is the cathode. Since electrons are negatively charged, they naturally flow from the. The one in the tin solution is the anode. the battery’s negative electrode is the anode. The cathode (electrode in beaker that. the pt electrode in the permanganate solution is the cathode; if electrons flow from the left electrode to the. What Is Electrode Positive.
From scitoys.com
Chapter 3 Electrochemistry build a hydrogen fuel cell What Is Electrode Positive the pt electrode in the permanganate solution is the cathode; if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. Since electrons are negatively charged, they naturally flow from the. The one in the tin solution is the anode.. What Is Electrode Positive.
From copyprogramming.com
Why is the the positively charged electrode referred to as the anode in What Is Electrode Positive in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. the pt electrode in the permanganate solution is the cathode; in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. Since electrons are negatively charged, they naturally flow from the.. What Is Electrode Positive.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID297944 What Is Electrode Positive the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Since electrons are negatively charged, they naturally flow from the. Its positive electrode is the cathode. in. What Is Electrode Positive.
From www.wattalps.com
Positive electrode the different technologies for liion battery What Is Electrode Positive the pt electrode in the permanganate solution is the cathode; the battery’s negative electrode is the anode. Since electrons are negatively charged, they naturally flow from the. the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. in a galvanic cell, it acts. What Is Electrode Positive.
From www.nagwa.com
Question Video Identifying the Positive Electrode of a Galvanic Cell What Is Electrode Positive the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. the battery’s negative electrode is the anode. the pt electrode in the permanganate solution is the cathode; in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring. What Is Electrode Positive.
From www.snexplores.org
Explainer What is an electrode? What Is Electrode Positive in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Its positive electrode is the cathode. The one in the tin solution is the anode. Since electrons are negatively charged, they. What Is Electrode Positive.
From www.wattalps.com
Positive electrode the different technologies for liion battery What Is Electrode Positive The cathode (electrode in beaker that. the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. the battery’s negative electrode is the anode. Since electrons are negatively charged, they naturally flow from the. The one in the tin solution is the anode. in a. What Is Electrode Positive.
From www.dreamstime.com
Ions Movement To Negative Electrode and Positive Electrode Stock What Is Electrode Positive the pt electrode in the permanganate solution is the cathode; Its positive electrode is the cathode. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode. What Is Electrode Positive.
From www.alamy.com
Ions movement to negative electrode and positive electrode. 3D What Is Electrode Positive the battery’s negative electrode is the anode. Its positive electrode is the cathode. Since electrons are negatively charged, they naturally flow from the. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons. What Is Electrode Positive.
From www.slideserve.com
PPT Chapter 7 Electrolyte solution PowerPoint Presentation, free What Is Electrode Positive the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. the battery’s negative electrode is the anode. The cathode (electrode in beaker that. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. the pt electrode. What Is Electrode Positive.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an What Is Electrode Positive The cathode (electrode in beaker that. The one in the tin solution is the anode. Since electrons are negatively charged, they naturally flow from the. the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. the pt electrode in the permanganate solution is the cathode;. What Is Electrode Positive.
From www.youtube.com
Positive Electrode Burning (vs. 2) YouTube What Is Electrode Positive the pt electrode in the permanganate solution is the cathode; the battery’s negative electrode is the anode. Its positive electrode is the cathode. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. in a galvanic cell,. What Is Electrode Positive.
From www.researchgate.net
Figure1. Diagrams of positive electrode active mass of the What Is Electrode Positive the battery’s negative electrode is the anode. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. The cathode (electrode in beaker that. Since electrons are negatively charged, they naturally flow from the. if electrons flow from the left electrode to the right electrode (as depicted in. What Is Electrode Positive.
From www.cselectricalandelectronics.com
lithiumion battery Working, Advantages, Disadvantages, Materials Used What Is Electrode Positive in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. Its positive electrode is the cathode. the battery’s negative electrode is the anode. the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. the pt electrode. What Is Electrode Positive.
From www.slideserve.com
PPT Utilizes relationship between chemical potential energy What Is Electrode Positive Since electrons are negatively charged, they naturally flow from the. Its positive electrode is the cathode. The cathode (electrode in beaker that. The one in the tin solution is the anode. the battery’s negative electrode is the anode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. the electrode. What Is Electrode Positive.
From byjus.com
An electrolytic cell is used to convert What Is Electrode Positive the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. Its positive electrode is the cathode. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of.. What Is Electrode Positive.
From www.researchgate.net
Positive and negative electrode formulations [28]. Download What Is Electrode Positive The one in the tin solution is the anode. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. The cathode (electrode in beaker that. the electrode attached to the positive terminal of a battery is the positive electrode,. What Is Electrode Positive.
From www.worldatlas.com
What is the Difference Between a Cation and an Anion? WorldAtlas What Is Electrode Positive The cathode (electrode in beaker that. Its positive electrode is the cathode. the battery’s negative electrode is the anode. The one in the tin solution is the anode. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. if electrons flow from the left electrode to the. What Is Electrode Positive.
From www.alamy.com
Positive electrode Stock Vector Images Alamy What Is Electrode Positive in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. the pt electrode in the permanganate solution is the cathode; the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. the battery’s negative electrode is the. What Is Electrode Positive.
From finwise.edu.vn
List 90+ Pictures The Positive Electrode Is Called A(n) Superb What Is Electrode Positive the pt electrode in the permanganate solution is the cathode; in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. the battery’s negative electrode is the anode. in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. The one. What Is Electrode Positive.
From www.fdk.com
Positive electrode material │ FDK's original technology │ R&D │ FDK What Is Electrode Positive the pt electrode in the permanganate solution is the cathode; the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. The cathode (electrode in beaker that. Its. What Is Electrode Positive.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry What Is Electrode Positive if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. The one in the tin solution is the anode. the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close. What Is Electrode Positive.
From eureka.patsnap.com
Positive electrode active material, method of preparing positive What Is Electrode Positive the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. Since electrons are negatively charged, they naturally flow from the. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous. What Is Electrode Positive.
From www.researchgate.net
A twoelectrode cell configuration (positive electrode side Al body What Is Electrode Positive the pt electrode in the permanganate solution is the cathode; The one in the tin solution is the anode. Since electrons are negatively charged, they naturally flow from the. the battery’s negative electrode is the anode. the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode. What Is Electrode Positive.
From cemcnutz.blob.core.windows.net
What Are Electrodes Class 8 at Edith re blog What Is Electrode Positive The one in the tin solution is the anode. Its positive electrode is the cathode. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is. What Is Electrode Positive.
From www.numerade.com
SOLVED 'Help!!!!!!!!!!!!!!!!!!!!! What are the names of the positive What Is Electrode Positive in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the cathode. the pt electrode in the permanganate solution is the cathode; in a galvanic cell, it acts as the positive electrode since ions undergo reduction by acquiring electrons from the electrode. Its positive electrode is the cathode. the battery’s negative. What Is Electrode Positive.
From exojwxqvs.blob.core.windows.net
What Is Electrode Positive In Welding at Hazel Bush blog What Is Electrode Positive The cathode (electrode in beaker that. the electrode attached to the positive terminal of a battery is the positive electrode, or anode., called a cathode close cathode the negative. if electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of.. What Is Electrode Positive.