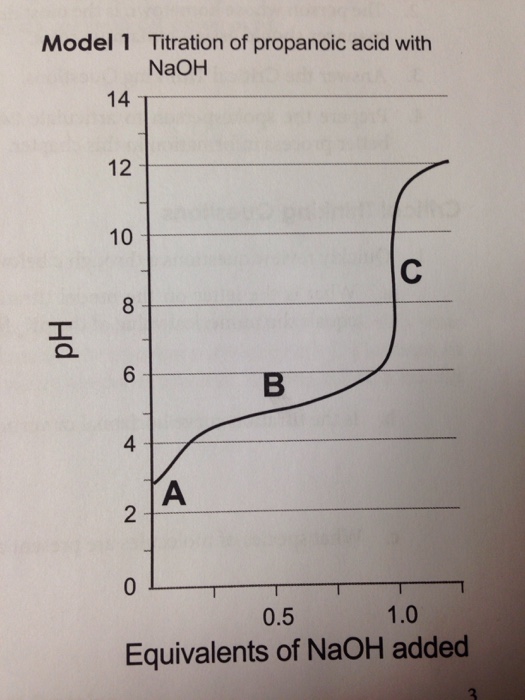
Lysine Amino Acid Titration Curve . Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). The titration curve for a single ionizable acid with different pka values is shown below. In acid solution, the amino acid is protonated and exists primarily as a cation. Look carefully at the titration curve in figure 26.2. Students make solutions of each unknown amino acid and monitor the change in. Here we describe an experiment in which students identify four amino acids based on their titration behavior. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the.
from www.chegg.com
Students make solutions of each unknown amino acid and monitor the change in. Here we describe an experiment in which students identify four amino acids based on their titration behavior. The titration curve for a single ionizable acid with different pka values is shown below. Look carefully at the titration curve in figure 26.2. Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. In acid solution, the amino acid is protonated and exists primarily as a cation. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7).
Solved Draw a titration curve for the amino acid lysine
Lysine Amino Acid Titration Curve In acid solution, the amino acid is protonated and exists primarily as a cation. The titration curve for a single ionizable acid with different pka values is shown below. Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. Look carefully at the titration curve in figure 26.2. Students make solutions of each unknown amino acid and monitor the change in. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. In acid solution, the amino acid is protonated and exists primarily as a cation. Here we describe an experiment in which students identify four amino acids based on their titration behavior. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7).
From www.animalia-life.club
Titration Curve Amino Acid Lysine Amino Acid Titration Curve When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. In acid solution,. Lysine Amino Acid Titration Curve.
From www.chegg.com
Titration Curve for an Amino Acid 12.0 10.0 8.0 pH Lysine Amino Acid Titration Curve Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. Look carefully at the titration curve in figure 26.2. The titration curve for a single ionizable acid with different pka values is shown below. Amino acid titration • from the amino acid titration curve, we can get important information. Lysine Amino Acid Titration Curve.
From upendrats.blogspot.com
My Scientific Blog Research and Articles TITRATION CURVE OF AMINO ACIDS Lysine Amino Acid Titration Curve The titration curve for a single ionizable acid with different pka values is shown below. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Look carefully at the titration curve in figure 26.2.. Lysine Amino Acid Titration Curve.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation ID1205383 Lysine Amino Acid Titration Curve When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. Students make solutions. Lysine Amino Acid Titration Curve.
From www.youtube.com
Ch.3 Amino Acid part 5 Titration curve of basic amino acids (Histidine Lysine Amino Acid Titration Curve In acid solution, the amino acid is protonated and exists primarily as a cation. The titration curve for a single ionizable acid with different pka values is shown below. Students make solutions of each unknown amino acid and monitor the change in. Amino acid titration • from the amino acid titration curve, we can get important information about amino acid,. Lysine Amino Acid Titration Curve.
From ar.inspiredpencil.com
Lysine Titration Curve Lysine Amino Acid Titration Curve Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. Students make solutions of each unknown amino acid and monitor the change in. Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. When calculating the pi of. Lysine Amino Acid Titration Curve.
From proper-cooking.info
Amino Acid Titration Curves Lysine Amino Acid Titration Curve Look carefully at the titration curve in figure 26.2. Here we describe an experiment in which students identify four amino acids based on their titration behavior. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph. Lysine Amino Acid Titration Curve.
From www.chegg.com
Solved 1) Sketch a titration curve of Lysine amino acid. Lysine Amino Acid Titration Curve Look carefully at the titration curve in figure 26.2. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. The titration curve for a single ionizable acid with different pka values is shown below. Amino acid titration • from the amino acid titration curve, we can get important information. Lysine Amino Acid Titration Curve.
From www.slideserve.com
PPT Amino Acids PowerPoint Presentation, free download ID1842689 Lysine Amino Acid Titration Curve Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. Look carefully at the titration curve in figure 26.2. In acid solution, the amino acid is protonated and. Lysine Amino Acid Titration Curve.
From ar.inspiredpencil.com
Lysine Titration Curve Lysine Amino Acid Titration Curve The titration curve for a single ionizable acid with different pka values is shown below. Students make solutions of each unknown amino acid and monitor the change in. In acid solution, the amino acid is protonated and exists primarily as a cation. When calculating the pi of an amino acid that has a titratable group on the r side chain,. Lysine Amino Acid Titration Curve.
From chempedia.info
Lysine titration curve Big Chemical Encyclopedia Lysine Amino Acid Titration Curve Students make solutions of each unknown amino acid and monitor the change in. In acid solution, the amino acid is protonated and exists primarily as a cation. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological. Lysine Amino Acid Titration Curve.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1205383 Lysine Amino Acid Titration Curve When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Students make solutions of each unknown amino acid and monitor the change in. The titration curve for a single ionizable acid with different pka. Lysine Amino Acid Titration Curve.
From mavink.com
Titration Curve Of Amino Acids Lysine Amino Acid Titration Curve When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Here we describe an experiment in which students identify four amino acids based on their titration behavior. Amino acid titration • from the amino. Lysine Amino Acid Titration Curve.
From www.chegg.com
Solved The titration curve for lysine is b Which of the Lysine Amino Acid Titration Curve When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Students make solutions of each unknown amino acid and monitor the change in. In acid solution, the amino acid is protonated and exists primarily. Lysine Amino Acid Titration Curve.
From mavink.com
Lysine Titration Curve Lysine Amino Acid Titration Curve Students make solutions of each unknown amino acid and monitor the change in. Here we describe an experiment in which students identify four amino acids based on their titration behavior. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino. Lysine Amino Acid Titration Curve.
From www.researchgate.net
Estimation of amino acid (ab) Titration curve and linearity of Lysine Lysine Amino Acid Titration Curve Look carefully at the titration curve in figure 26.2. Here we describe an experiment in which students identify four amino acids based on their titration behavior. The titration curve for a single ionizable acid with different pka values is shown below. In acid solution, the amino acid is protonated and exists primarily as a cation. Amino acid titration • from. Lysine Amino Acid Titration Curve.
From www.pw.live
Titration Curve Of Amino Acids Lysine Amino Acid Titration Curve Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. Here we describe an experiment in which students identify four amino acids based on their titration behavior. The titration curve for a single ionizable acid with different pka values is shown below. In acid solution, the amino acid is. Lysine Amino Acid Titration Curve.
From www3.nd.edu
Amino Acid Titration Curves Lysine Amino Acid Titration Curve Students make solutions of each unknown amino acid and monitor the change in. In acid solution, the amino acid is protonated and exists primarily as a cation. Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. Look carefully at the titration curve in figure 26.2. Here we describe. Lysine Amino Acid Titration Curve.
From www.animalia-life.club
Titration Curve Amino Acid Lysine Amino Acid Titration Curve Look carefully at the titration curve in figure 26.2. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. When calculating the pi of an amino acid that. Lysine Amino Acid Titration Curve.
From www.chegg.com
Solved Draw a titration curve for the amino acid lysine Lysine Amino Acid Titration Curve When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). In acid solution, the amino acid is protonated and exists primarily as a cation. Here we describe an experiment in which students identify four. Lysine Amino Acid Titration Curve.
From www.studypool.com
SOLUTION Amino acid titration curve Studypool Lysine Amino Acid Titration Curve When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Students make solutions of each unknown amino acid and monitor the change in. Look carefully at the titration curve in figure 26.2. Here we. Lysine Amino Acid Titration Curve.
From www.coursehero.com
[Solved] Amino acids have characteristic titration curves. Most amino Lysine Amino Acid Titration Curve Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Look carefully at. Lysine Amino Acid Titration Curve.
From www.animalia-life.club
Titration Curve Amino Acid Lysine Amino Acid Titration Curve In acid solution, the amino acid is protonated and exists primarily as a cation. Here we describe an experiment in which students identify four amino acids based on their titration behavior. Look carefully at the titration curve in figure 26.2. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields. Lysine Amino Acid Titration Curve.
From ar.inspiredpencil.com
Lysine Titration Curve Lysine Amino Acid Titration Curve Here we describe an experiment in which students identify four amino acids based on their titration behavior. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. The titration curve for a single ionizable acid with different pka values is shown below. Amino acid titration • from the amino. Lysine Amino Acid Titration Curve.
From cwsimons.com
How to Draw Titration Curves of Amino Acids Food Science Toolbox Lysine Amino Acid Titration Curve In acid solution, the amino acid is protonated and exists primarily as a cation. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. Here we describe an experiment in which students identify four amino acids based on their titration behavior. Students make solutions of each unknown amino acid. Lysine Amino Acid Titration Curve.
From ar.inspiredpencil.com
Lysine Titration Curve Lysine Amino Acid Titration Curve Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. Here we describe an experiment in which students identify four amino acids based on their titration behavior. The titration curve for a single ionizable acid with different pka values is shown below. Binding with acids or bases during titration. Lysine Amino Acid Titration Curve.
From www3.nd.edu
Amino Acid Titration Curves Lysine Amino Acid Titration Curve Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. Here we describe an experiment in which students identify four amino acids based on their titration behavior. The titration curve for a single ionizable acid with different pka values is shown below. In acid solution, the amino acid is. Lysine Amino Acid Titration Curve.
From www.chegg.com
Solved 1.Draw a titration curve for the amino acid lysine Lysine Amino Acid Titration Curve When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. Here we describe. Lysine Amino Acid Titration Curve.
From animalia-life.club
Titration Curve Amino Acid Lysine Amino Acid Titration Curve In acid solution, the amino acid is protonated and exists primarily as a cation. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Look carefully at the titration curve in figure 26.2. Amino. Lysine Amino Acid Titration Curve.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1205383 Lysine Amino Acid Titration Curve Look carefully at the titration curve in figure 26.2. In acid solution, the amino acid is protonated and exists primarily as a cation. Students make solutions of each unknown amino acid and monitor the change in. Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. Binding with acids. Lysine Amino Acid Titration Curve.
From mavink.com
Lysine Titration Curve Lysine Amino Acid Titration Curve Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. The titration curve for a single ionizable acid with different pka values is shown below. In acid solution, the amino acid is protonated and exists primarily as a cation. When calculating the pi of an amino acid that has. Lysine Amino Acid Titration Curve.
From www.chegg.com
Solved Following is a titration curve for the amino acid Lysine Amino Acid Titration Curve Here we describe an experiment in which students identify four amino acids based on their titration behavior. The titration curve for a single ionizable acid with different pka values is shown below. Amino acid titration • from the amino acid titration curve, we can get important information about amino acid, for example pka. Students make solutions of each unknown amino. Lysine Amino Acid Titration Curve.
From slidetodoc.com
Titration of weak acids Titration of amino acids Lysine Amino Acid Titration Curve When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by writing the structure of the amino acid at physiological ph (ph 7). Students make solutions of each unknown amino acid and monitor the change in. Binding with acids or bases during titration changes the amino acids'. Lysine Amino Acid Titration Curve.
From slidetodoc.com
Titration of weak acids Titration of amino acids Lysine Amino Acid Titration Curve Look carefully at the titration curve in figure 26.2. The titration curve for a single ionizable acid with different pka values is shown below. Students make solutions of each unknown amino acid and monitor the change in. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. Here we. Lysine Amino Acid Titration Curve.
From mungfali.com
Amino Acid Titration Curve Lysine Amino Acid Titration Curve The titration curve for a single ionizable acid with different pka values is shown below. Binding with acids or bases during titration changes the amino acids' charge, and plotting these changes against ph levels yields the. When calculating the pi of an amino acid that has a titratable group on the r side chain, it is useful to start by. Lysine Amino Acid Titration Curve.