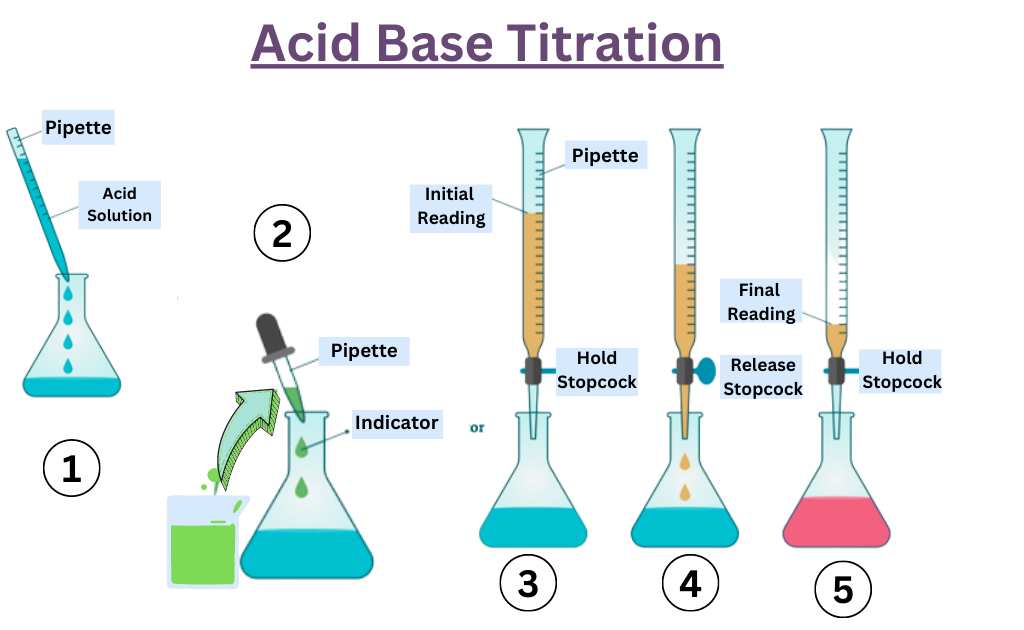
Titration Reagent Definition . Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. This titration technique relies on a chemical. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent.
from themasterchemistry.com
Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This titration technique relies on a chemical.
Acid Base TitrationWorking Principle, Process, Types And Indicators
Titration Reagent Definition Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This titration technique relies on a chemical. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Reagent Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is the slow addition of one. Titration Reagent Definition.
From www.chegg.com
Solved Definition The point in a titration when the added Titration Reagent Definition Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine the concentration of an unknown solution by. Titration Reagent Definition.
From joioqtatt.blob.core.windows.net
Types Of Titration Reactions at Alex Ortiz blog Titration Reagent Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content. Titration Reagent Definition.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Reagent Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. This titration technique relies on a chemical. A titration is a. Titration Reagent Definition.
From www.alamy.com
Titration experiment, adding chemical of known concentration to test Titration Reagent Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. This titration technique relies on a chemical. A titration is a. Titration Reagent Definition.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Reagent Definition This titration technique relies on a chemical. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Karl fischer titration is a widely utilized analytical method. Titration Reagent Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Reagent Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as. Titration Reagent Definition.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Reagent Definition This titration technique relies on a chemical. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known. Titration Reagent Definition.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Titration Reagent Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called. Titration Reagent Definition.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Reagent Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used. Titration Reagent Definition.
From www.vectorstock.com
Acid base titration experiment and phases Vector Image Titration Reagent Definition Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. This titration technique relies on a chemical. Titration is the slow addition of one solution of a known. Titration Reagent Definition.
From mavink.com
Titration Diagram Labeled Titration Reagent Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. This titration technique relies on a chemical. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration involves the gradual addition of a. Titration Reagent Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Reagent Definition Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Titration Reagent Definition.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Reagent Definition Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. This titration technique relies on a chemical. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is the slow addition of one. Titration Reagent Definition.
From scienceinfo.com
Titration Definition, 4 Types, Procedure Titration Reagent Definition Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. This titration technique relies on a chemical. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. A titration is a. Titration Reagent Definition.
From www.slideserve.com
PPT Titration PowerPoint Presentation, free download ID5970774 Titration Reagent Definition This titration technique relies on a chemical. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is an analytical. Titration Reagent Definition.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID Titration Reagent Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration, process of chemical analysis in which the quantity of some constituent of a. Titration Reagent Definition.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Reagent Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Titration Reagent Definition.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Reagent Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Karl fischer titration is a widely utilized analytical method specifically designed. Titration Reagent Definition.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Reagent Definition Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. This titration technique relies on a chemical. Titration involves the gradual addition of a reagent of known concentration,. Titration Reagent Definition.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Reagent Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Karl fischer titration is a widely utilized. Titration Reagent Definition.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Titration Reagent Definition Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. This titration technique relies on a chemical. Titration is the slow addition of. Titration Reagent Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Reagent Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration, process of chemical analysis in which the quantity of some constituent of. Titration Reagent Definition.
From www.chegg.com
Solved Definition The point in a titration when the added Titration Reagent Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. This titration technique relies on a chemical. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the.. Titration Reagent Definition.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Titration Reagent Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. This titration technique relies on a chemical. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of. Titration Reagent Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Reagent Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is an analytical technique that allows the quantitative determination of a specific. Titration Reagent Definition.
From sciencenotes.org
What Is a Primary Standard in Chemistry? Titration Reagent Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration is the slow addition of one solution of a known concentration (called a titrant) to a. Titration Reagent Definition.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation Titration Reagent Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. This titration technique relies on a chemical. Titration, process of chemical. Titration Reagent Definition.
From gamesmartz.com
Titration Definition & Image GameSmartz Titration Reagent Definition Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. This titration technique relies on a chemical. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration is the slow. Titration Reagent Definition.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration Reagent Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration Reagent Definition.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Reagent Definition This titration technique relies on a chemical. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration is a quantitative analysis to determine the concentration of an. Titration Reagent Definition.
From celtjcvb.blob.core.windows.net
Titration In Simple Terms at John Haslett blog Titration Reagent Definition Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This titration technique relies on a chemical. Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration, process of chemical analysis in which the quantity of. Titration Reagent Definition.
From www.savemyexams.co.uk
Titrations (1.7.2) Edexcel A Level Chemistry Revision Notes 2017 Titration Reagent Definition Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used to precisely measure. Titration Reagent Definition.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Titration Reagent Definition A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. Titration Reagent Definition.
From www.sliderbase.com
Titration Titration Reagent Definition Karl fischer titration is a widely utilized analytical method specifically designed to determine the moisture content in various substances. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine the concentration of an unknown solution by. Titration Reagent Definition.