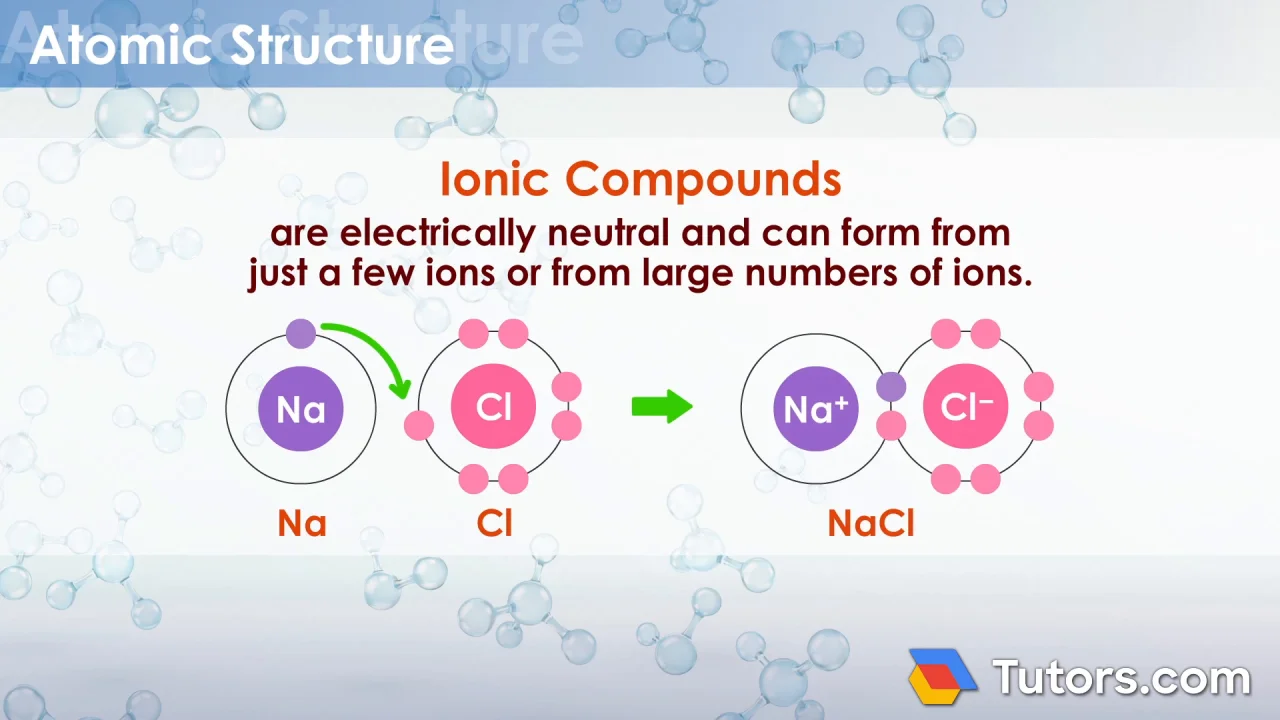
Brittle Ionic Compounds Examples With Oxygen . An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. Solutions of ionic compounds and melted ionic compounds. By the end of this section, you will be able to: What happens when an electric current is passed. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Ionic compounds dissociate into ions when dissolved in water. Why are melting points high for ionic compounds? Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Explain the formation of cations, anions, and ionic compounds. Ionic compounds form when atoms connect to one another by ionic bonds. If the electronegativity difference is 2 or more, the elements form. Why are ionic compounds brittle? Predict the charge of common. Ionic solids are held together by the electrostatic attraction. Ionic compounds are hard and brittle.
from www.animalia-life.club
What happens when an electric current is passed. Solutions of ionic compounds and melted ionic compounds. If the electronegativity difference is 2 or more, the elements form. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Why are melting points high for ionic compounds? Ionic compounds dissociate into ions when dissolved in water. Why are ionic compounds brittle? Predict the charge of common. By the end of this section, you will be able to: Ionic solids are held together by the electrostatic attraction.
Properties Of Ionic Compounds
Brittle Ionic Compounds Examples With Oxygen What happens when an electric current is passed. Why are melting points high for ionic compounds? Solutions of ionic compounds and melted ionic compounds. Predict the charge of common. If the electronegativity difference is 2 or more, the elements form. Why are ionic compounds brittle? An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Ionic compounds form when atoms connect to one another by ionic bonds. Explain the formation of cations, anions, and ionic compounds. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Ionic solids are held together by the electrostatic attraction. By the end of this section, you will be able to: What happens when an electric current is passed. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds.
From mavink.com
5 Examples Of Ionic Compounds Brittle Ionic Compounds Examples With Oxygen Why are melting points high for ionic compounds? Why are ionic compounds brittle? What happens when an electric current is passed. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Solutions of ionic compounds and melted ionic compounds. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority. Brittle Ionic Compounds Examples With Oxygen.
From www.chemistrylearner.com
Ionic Bond Facts, Definition, Properties, Examples, & Diagrams Brittle Ionic Compounds Examples With Oxygen If the electronegativity difference is 2 or more, the elements form. What happens when an electric current is passed. Ionic compounds are hard and brittle. Predict the charge of common. Explain the formation of cations, anions, and ionic compounds. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. An ionic bond is the strongest type of. Brittle Ionic Compounds Examples With Oxygen.
From slideplayer.com
Ionic Bond Chapter 5 Section ppt download Brittle Ionic Compounds Examples With Oxygen Ionic solids are held together by the electrostatic attraction. Predict the charge of common. Ionic compounds form when atoms connect to one another by ionic bonds. Ionic compounds are hard and brittle. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Explain the formation of cations, anions, and. Brittle Ionic Compounds Examples With Oxygen.
From ibstudy.net
4.1 Ionic bonding and structure Wang's website Brittle Ionic Compounds Examples With Oxygen As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. If the electronegativity difference is 2 or more, the elements form. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Why are ionic compounds brittle? Why are melting points high for ionic compounds? Solutions of. Brittle Ionic Compounds Examples With Oxygen.
From www.slideshare.net
Ionic bonding Brittle Ionic Compounds Examples With Oxygen What happens when an electric current is passed. Predict the charge of common. By the end of this section, you will be able to: Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Explain the formation of cations, anions, and ionic compounds. Why are ionic compounds brittle? Ionic compounds are hard and brittle. An ionic bond. Brittle Ionic Compounds Examples With Oxygen.
From www.britannica.com
Chemical compound Definition, Examples, & Types Britannica Brittle Ionic Compounds Examples With Oxygen Solutions of ionic compounds and melted ionic compounds. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Predict the charge of common. Ionic compounds form when atoms connect to one another by ionic bonds. An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. If the electronegativity difference is 2 or. Brittle Ionic Compounds Examples With Oxygen.
From www.slideserve.com
PPT BC Science Connections 9 Unit 2 The electron arrangement of Brittle Ionic Compounds Examples With Oxygen Predict the charge of common. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Solutions of ionic compounds and melted ionic compounds. Ionic solids are held together by the electrostatic attraction. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Why are ionic compounds. Brittle Ionic Compounds Examples With Oxygen.
From slideplayer.com
Ch. 7 Ionic Bonds. ppt download Brittle Ionic Compounds Examples With Oxygen Ionic solids are held together by the electrostatic attraction. An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. By the end of this section, you will be able to: Why are ionic compounds. Brittle Ionic Compounds Examples With Oxygen.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Brittle Ionic Compounds Examples With Oxygen Explain the formation of cations, anions, and ionic compounds. Ionic solids are held together by the electrostatic attraction. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Why are ionic compounds brittle? What happens when an electric current is passed. Why are melting points high for ionic compounds? Ionic compounds are hard and brittle. Ionic compounds. Brittle Ionic Compounds Examples With Oxygen.
From www.slideserve.com
PPT Properties of ionic compounds PowerPoint Presentation ID1130121 Brittle Ionic Compounds Examples With Oxygen If the electronegativity difference is 2 or more, the elements form. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Explain the formation of cations, anions, and ionic compounds. Why are melting points high for ionic compounds? Why are ionic compounds brittle? Ionic solids are held together by. Brittle Ionic Compounds Examples With Oxygen.
From slideplayer.com
Ionic and Covalent Bonds ppt download Brittle Ionic Compounds Examples With Oxygen Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Why are ionic compounds brittle? Ionic compounds dissociate into ions when dissolved in water. Ionic compounds form when atoms connect to one another by ionic bonds. An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. Solutions of ionic compounds and melted. Brittle Ionic Compounds Examples With Oxygen.
From slideplayer.com
Ionic & Metallic Bonding ppt download Brittle Ionic Compounds Examples With Oxygen Why are melting points high for ionic compounds? As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. By the end of this section, you will be able to: Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. If the electronegativity difference is 2 or. Brittle Ionic Compounds Examples With Oxygen.
From study.com
Chemical Formula for Ionic Compound Binary & Polyatomic Lesson Brittle Ionic Compounds Examples With Oxygen Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Why are ionic compounds brittle? Why are melting points high for ionic compounds? By the end of this section, you will be able to: If the. Brittle Ionic Compounds Examples With Oxygen.
From www.youtube.com
Examples of Ionic Compoiunds YouTube Brittle Ionic Compounds Examples With Oxygen Ionic compounds form when atoms connect to one another by ionic bonds. Ionic compounds dissociate into ions when dissolved in water. Explain the formation of cations, anions, and ionic compounds. What happens when an electric current is passed. Ionic solids are held together by the electrostatic attraction. An ionic bond is the strongest type of chemical bond, which leads to. Brittle Ionic Compounds Examples With Oxygen.
From tristianaresshepard.blogspot.com
Oxygen Chemical Formula TristianaresShepard Brittle Ionic Compounds Examples With Oxygen Ionic compounds form when atoms connect to one another by ionic bonds. Predict the charge of common. By the end of this section, you will be able to: What happens when an electric current is passed. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Explain the formation. Brittle Ionic Compounds Examples With Oxygen.
From www.youtube.com
Why ionic solids are brittle? YouTube Brittle Ionic Compounds Examples With Oxygen Ionic solids are held together by the electrostatic attraction. What happens when an electric current is passed. By the end of this section, you will be able to: An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. Why are melting points high for ionic compounds? Explain the formation of cations, anions, and ionic compounds.. Brittle Ionic Compounds Examples With Oxygen.
From slidetodoc.com
TYPES OF CHEMICAL BONDS IONIC BONDS COVALENT BONDS Brittle Ionic Compounds Examples With Oxygen Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Ionic compounds are hard and brittle. Predict the charge of common. Ionic compounds form when atoms connect to one another by ionic bonds. Explain the formation of cations, anions, and ionic compounds. Ionic compounds dissociate into ions when dissolved in water. As a general rule, if the. Brittle Ionic Compounds Examples With Oxygen.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Brittle Ionic Compounds Examples With Oxygen Solutions of ionic compounds and melted ionic compounds. Ionic compounds are hard and brittle. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. If the electronegativity difference is 2 or more, the elements form. By the end of this section, you will be able to: Ionic compounds include. Brittle Ionic Compounds Examples With Oxygen.
From sciencenotes.org
Ionic Bond Definition and Examples Brittle Ionic Compounds Examples With Oxygen Why are ionic compounds brittle? Predict the charge of common. If the electronegativity difference is 2 or more, the elements form. Solutions of ionic compounds and melted ionic compounds. Why are melting points high for ionic compounds? Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Ionic compounds form when atoms connect to one another by. Brittle Ionic Compounds Examples With Oxygen.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Brittle Ionic Compounds Examples With Oxygen What happens when an electric current is passed. If the electronegativity difference is 2 or more, the elements form. By the end of this section, you will be able to: Why are melting points high for ionic compounds? Solutions of ionic compounds and melted ionic compounds. As a general rule, if the electronegativity difference is less than 2 on the. Brittle Ionic Compounds Examples With Oxygen.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Brittle Ionic Compounds Examples With Oxygen Ionic solids are held together by the electrostatic attraction. Ionic compounds are hard and brittle. Ionic compounds form when atoms connect to one another by ionic bonds. Ionic compounds dissociate into ions when dissolved in water. An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. As a general rule, if the electronegativity difference is. Brittle Ionic Compounds Examples With Oxygen.
From ar.inspiredpencil.com
Ionic Compounds Examples Brittle Ionic Compounds Examples With Oxygen Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Why are ionic compounds brittle? Why are melting points high for ionic compounds? Ionic compounds are hard and brittle. If the electronegativity difference is 2 or more, the elements form. Predict the charge of common. Solutions of ionic compounds and melted ionic compounds. Explain the formation of. Brittle Ionic Compounds Examples With Oxygen.
From nzohomeworkuht.web.fc2.com
How to write ionic formulas for compounds Brittle Ionic Compounds Examples With Oxygen If the electronegativity difference is 2 or more, the elements form. An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. By the end of this section, you will be able to: Predict the charge of common. Explain the formation of cations, anions, and ionic compounds. Ionic solids are held together by the electrostatic attraction.. Brittle Ionic Compounds Examples With Oxygen.
From www.animalia-life.club
Properties Of Ionic Compounds Brittle Ionic Compounds Examples With Oxygen An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. Why are ionic compounds brittle? Ionic compounds dissociate into ions when dissolved in water. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Explain the formation of cations, anions, and ionic compounds. Ionic. Brittle Ionic Compounds Examples With Oxygen.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination Brittle Ionic Compounds Examples With Oxygen If the electronegativity difference is 2 or more, the elements form. Solutions of ionic compounds and melted ionic compounds. Predict the charge of common. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. By the end of this section, you will be able to: An ionic bond is the strongest type of chemical bond, which leads. Brittle Ionic Compounds Examples With Oxygen.
From slideplayer.com
Chemical Bonding Chapter ppt download Brittle Ionic Compounds Examples With Oxygen Explain the formation of cations, anions, and ionic compounds. An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Solutions of ionic compounds and melted ionic compounds. Ionic compounds dissociate into ions when dissolved. Brittle Ionic Compounds Examples With Oxygen.
From ar.inspiredpencil.com
Ionic Compound List Brittle Ionic Compounds Examples With Oxygen Solutions of ionic compounds and melted ionic compounds. Ionic solids are held together by the electrostatic attraction. Ionic compounds are hard and brittle. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. What happens when an electric current is passed. Ionic compounds dissociate into ions when dissolved in water. An ionic bond is the strongest type. Brittle Ionic Compounds Examples With Oxygen.
From slideplayer.com
Ionic Bonding/Naming. ppt download Brittle Ionic Compounds Examples With Oxygen An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. If the electronegativity difference is 2 or more, the elements form. By the end of this section, you will be able to: Explain the formation of cations, anions, and ionic compounds. Predict the charge of common. Ionic compounds include salts, oxides, hydroxides, sulphides, and the. Brittle Ionic Compounds Examples With Oxygen.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Brittle Ionic Compounds Examples With Oxygen An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. Why are ionic compounds brittle? Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. What happens when an electric current is passed. Ionic compounds dissociate into ions when dissolved in water. By the end of this section, you will be able. Brittle Ionic Compounds Examples With Oxygen.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Brittle Ionic Compounds Examples With Oxygen As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Predict the charge of common. By the end of this section, you will be able to: Explain the formation of cations, anions, and ionic compounds. Solutions of ionic compounds and melted ionic compounds. What happens when an electric current. Brittle Ionic Compounds Examples With Oxygen.
From brunofuga.adv.br
Ionic Compounds Definition, Properties, Examples, 46 OFF Brittle Ionic Compounds Examples With Oxygen An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. Solutions of ionic compounds and melted ionic compounds. Explain the formation of cations, anions, and ionic compounds. Ionic compounds dissociate into ions when dissolved in water. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form. Brittle Ionic Compounds Examples With Oxygen.
From ar.inspiredpencil.com
Ionic Compounds Examples Brittle Ionic Compounds Examples With Oxygen Ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. Why are ionic compounds brittle? As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. An ionic bond is the strongest type of chemical bond, which leads to characteristic properties. What happens when an. Brittle Ionic Compounds Examples With Oxygen.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Brittle Ionic Compounds Examples With Oxygen Predict the charge of common. Ionic compounds form when atoms connect to one another by ionic bonds. As a general rule, if the electronegativity difference is less than 2 on the pauling scale, the atoms form covalent bonds. Why are melting points high for ionic compounds? Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. What. Brittle Ionic Compounds Examples With Oxygen.
From slideplayer.com
Chemistry Ionic Compounds. ppt download Brittle Ionic Compounds Examples With Oxygen Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. By the end of this section, you will be able to: Ionic compounds are hard and brittle. Predict the charge of common. What happens when an electric current is passed. Ionic compounds dissociate into ions when dissolved in water. Ionic solids are held together by the electrostatic. Brittle Ionic Compounds Examples With Oxygen.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Brittle Ionic Compounds Examples With Oxygen Why are ionic compounds brittle? Predict the charge of common. What happens when an electric current is passed. Ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. By the end of this section, you will be able to: If the electronegativity difference is 2 or more, the elements form. Why are melting points high for ionic. Brittle Ionic Compounds Examples With Oxygen.