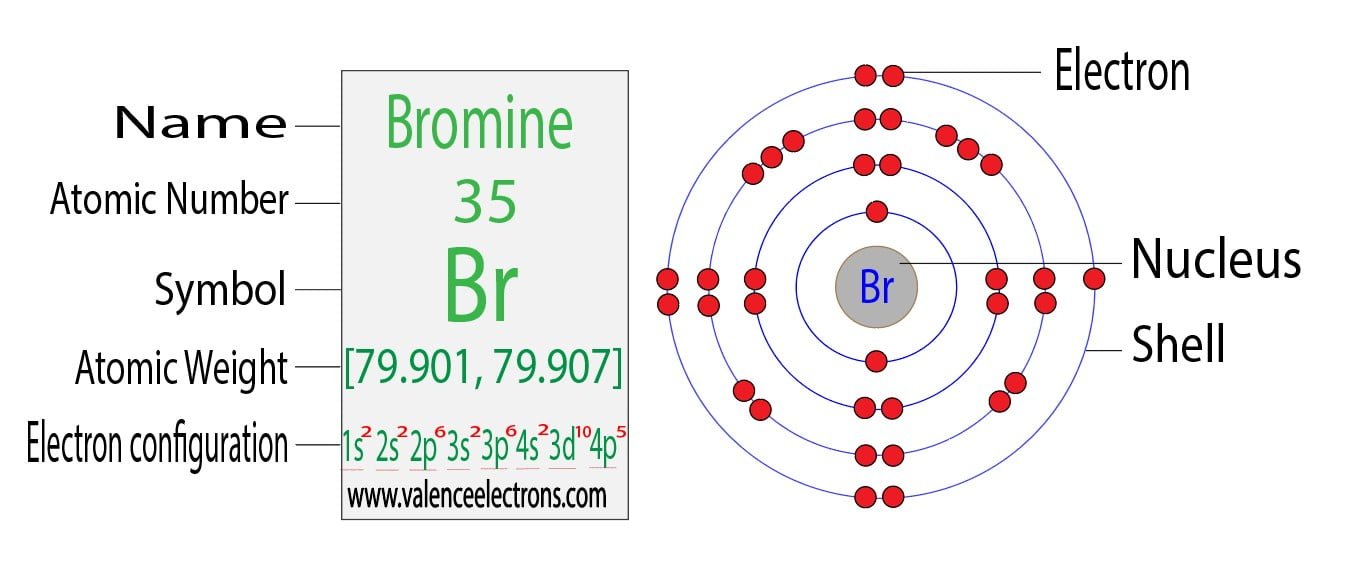
Bromine Has 28 Core Electrons . In this article today we are going to tell you about the electron configuration of bromine, its orbital. Electron configuration chart of all elements is mentioned in the table below. 1 determine the atomic number of bromine (br), which is 35. The shorthand electron configuration (or noble gas configuration) as well as full electron. Bromine (br) has 35 electrons in total. Like all halogens, it is thus one electron short of. This means a neutral bromine atom has 35 electrons. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. But for most of the transition. Bromine is the 35th element in the periodic table and the symbol is ‘br’. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells).
from www.animalia-life.club
Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine is the 35th element in the periodic table and the symbol is ‘br’. The shorthand electron configuration (or noble gas configuration) as well as full electron. In this article today we are going to tell you about the electron configuration of bromine, its orbital. But for most of the transition. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. This means a neutral bromine atom has 35 electrons. Electron configuration chart of all elements is mentioned in the table below. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells).
Electron Configuration For Bromine
Bromine Has 28 Core Electrons Like all halogens, it is thus one electron short of. Like all halogens, it is thus one electron short of. But for most of the transition. Bromine (br) has 35 electrons in total. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). In this article today we are going to tell you about the electron configuration of bromine, its orbital. The shorthand electron configuration (or noble gas configuration) as well as full electron. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Electron configuration chart of all elements is mentioned in the table below. 1 determine the atomic number of bromine (br), which is 35. This means a neutral bromine atom has 35 electrons. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Bromine is the 35th element in the periodic table and the symbol is ‘br’.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has 28 Core Electrons Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. But for most of the transition. This means a neutral bromine atom has 35 electrons. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Bromine. Bromine Has 28 Core Electrons.
From www.transtutors.com
(Get Answer) The Elements In Group 2A Are Known By What Name? Alkali Bromine Has 28 Core Electrons 1 determine the atomic number of bromine (br), which is 35. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Like all halogens, it is thus one electron short of. It has 7 valence electrons (outermost shell). Bromine Has 28 Core Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has 28 Core Electrons But for most of the transition. Bromine is the 35th element in the periodic table and the symbol is ‘br’. In this article today we are going to tell you about the electron configuration of bromine, its orbital. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting. Bromine Has 28 Core Electrons.
From lambdageeks.com
Bromine Electron Configuration 7 Easy Steps on How to Write Bromine Has 28 Core Electrons The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. But for most of the transition. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). Bromine has an atomic. Bromine Has 28 Core Electrons.
From www.numerade.com
SOLVED How many core electrons does a bromine atom have? 28 0 7 8 17 10 Bromine Has 28 Core Electrons Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). But for most of the transition. Like all halogens, it is thus one electron short of. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with. Bromine Has 28 Core Electrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Has 28 Core Electrons The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine (br) has 35 electrons in total. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its. Bromine Has 28 Core Electrons.
From www.vrogue.co
Bromine Electron Configuration Br With Orbital Diagra vrogue.co Bromine Has 28 Core Electrons The shorthand electron configuration (or noble gas configuration) as well as full electron. This means a neutral bromine atom has 35 electrons. Bromine (br) has 35 electrons in total. Electron configuration chart of all elements is mentioned in the table below. 1 determine the atomic number of bromine (br), which is 35. It has 7 valence electrons (outermost shell) and. Bromine Has 28 Core Electrons.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Has 28 Core Electrons Like all halogens, it is thus one electron short of. The shorthand electron configuration (or noble gas configuration) as well as full electron. This means a neutral bromine atom has 35 electrons. But for most of the transition. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p. Bromine Has 28 Core Electrons.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine Has 28 Core Electrons Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. But for most of the transition. Bromine is the 35th element in the periodic table and the symbol is ‘br’. 1 determine the atomic number of bromine (br), which is 35. In this article. Bromine Has 28 Core Electrons.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and Bromine Has 28 Core Electrons Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Electron configuration. Bromine Has 28 Core Electrons.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Bromine Has 28 Core Electrons 1 determine the atomic number of bromine (br), which is 35. But for most of the transition. In this article today we are going to tell you about the electron configuration of bromine, its orbital. The shorthand electron configuration (or noble gas configuration) as well as full electron. Bromine (br) has 35 electrons in total. Bromine has an atomic number. Bromine Has 28 Core Electrons.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine Has 28 Core Electrons Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. In this article today we are going to tell you about the electron configuration of bromine, its orbital. This means. Bromine Has 28 Core Electrons.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration Bromine Has 28 Core Electrons Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. 1 determine the atomic number of bromine (br), which is 35. Like all halogens, it is thus one electron short of. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2. Bromine Has 28 Core Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has 28 Core Electrons But for most of the transition. 1 determine the atomic number of bromine (br), which is 35. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5,. Bromine Has 28 Core Electrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Has 28 Core Electrons In this article today we are going to tell you about the electron configuration of bromine, its orbital. Like all halogens, it is thus one electron short of. Electron configuration chart of all elements is mentioned in the table below. 1 determine the atomic number of bromine (br), which is 35. The shorthand electron configuration (or noble gas configuration) as. Bromine Has 28 Core Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has 28 Core Electrons Bromine (br) has 35 electrons in total. 1 determine the atomic number of bromine (br), which is 35. Bromine is the 35th element in the periodic table and the symbol is ‘br’. In this article today we are going to tell you about the electron configuration of bromine, its orbital. Electron configuration chart of all elements is mentioned in the. Bromine Has 28 Core Electrons.
From www.numerade.com
SOLVED For the Bromine atom a) Determine the total number of unpaired Bromine Has 28 Core Electrons Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Bromine (br) has 35 electrons in total. Bromine is the 35th element in the periodic table and the symbol is ‘br’. This means a neutral bromine atom has 35 electrons. The shorthand electron configuration. Bromine Has 28 Core Electrons.
From www.dreamstime.com
Atom of Bromine with Detailed Core and Its 35 Electrons on Black Stock Bromine Has 28 Core Electrons Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine (br) has 35 electrons in. Bromine Has 28 Core Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Has 28 Core Electrons In this article today we are going to tell you about the electron configuration of bromine, its orbital. Bromine (br) has 35 electrons in total. But for most of the transition. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). The shorthand electron configuration (or noble gas configuration) as well as full electron. Bromine is. Bromine Has 28 Core Electrons.
From ar.inspiredpencil.com
Bromine Valence Electrons Bromine Has 28 Core Electrons Bromine (br) has 35 electrons in total. Like all halogens, it is thus one electron short of. Electron configuration chart of all elements is mentioned in the table below. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Bromine is the 35th element. Bromine Has 28 Core Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Has 28 Core Electrons It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). The shorthand electron configuration (or noble gas configuration) as well as full electron. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement. Bromine Has 28 Core Electrons.
From slideplayer.com
ANSWERS to Activity Qs 113 (pg 7071) ppt download Bromine Has 28 Core Electrons The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. This means a neutral bromine atom has 35 electrons. Bromine has an atomic number of 35, which means that its atom has 35 electrons. Bromine Has 28 Core Electrons.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Has 28 Core Electrons Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). But for most of the transition. Bromine (br) has 35 electrons in total. This means a neutral bromine atom has. Bromine Has 28 Core Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has 28 Core Electrons This means a neutral bromine atom has 35 electrons. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Like all halogens, it is thus one electron short of. 1 determine the atomic number of bromine (br), which. Bromine Has 28 Core Electrons.
From www.slideserve.com
PPT Topic Chemistry Aim Explain how elements are classified in Bromine Has 28 Core Electrons 1 determine the atomic number of bromine (br), which is 35. Bromine is the 35th element in the periodic table and the symbol is ‘br’. The shorthand electron configuration (or noble gas configuration) as well as full electron. Electron configuration chart of all elements is mentioned in the table below. Bromine (br) has 35 electrons in total. In this article. Bromine Has 28 Core Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Has 28 Core Electrons Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s. Bromine Has 28 Core Electrons.
From www.bartleby.com
Answered How many core electrons does the… bartleby Bromine Has 28 Core Electrons Like all halogens, it is thus one electron short of. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). Electron configuration chart of all elements is mentioned in the table below. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. This means a neutral bromine atom. Bromine Has 28 Core Electrons.
From www.britannica.com
Bromine Properties, Uses, & Facts Britannica Bromine Has 28 Core Electrons Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Bromine has an atomic number of 35, which means that its atom has 35 electrons around its nucleus. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s. Bromine Has 28 Core Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Has 28 Core Electrons 1 determine the atomic number of bromine (br), which is 35. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). This means a neutral bromine atom has 35 electrons. The shorthand electron configuration (or noble gas configuration) as well as full electron. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or. Bromine Has 28 Core Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has 28 Core Electrons The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. In this article today we are going to tell you about the electron configuration of bromine, its orbital. The shorthand electron configuration (or noble. Bromine Has 28 Core Electrons.
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide Bromine Has 28 Core Electrons But for most of the transition. Bromine (br) has 35 electrons in total. The shorthand electron configuration (or noble gas configuration) as well as full electron. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. It has 7 valence electrons (outermost shell) and. Bromine Has 28 Core Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has 28 Core Electrons The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within. Bromine (br) has 35 electrons in total. Like all halogens, it is thus one electron short of. Bromine is the 35th element in the. Bromine Has 28 Core Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Has 28 Core Electrons In this article today we are going to tell you about the electron configuration of bromine, its orbital. Like all halogens, it is thus one electron short of. The bromine electron configuration, denoted as [ar] 4s 2 3d 10 4p 5or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise. Bromine Has 28 Core Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has 28 Core Electrons Like all halogens, it is thus one electron short of. Bromine has the electron configuration [ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Electron configuration chart of all elements is mentioned in the table below. Bromine (br) has 35 electrons in total. It has 7 valence electrons. Bromine Has 28 Core Electrons.
From www.dreamstime.com
Element of Bromine stock vector. Illustration of college 104400483 Bromine Has 28 Core Electrons Electron configuration chart of all elements is mentioned in the table below. It has 7 valence electrons (outermost shell) and 28 core electrons (inner electron shells). Bromine is the 35th element in the periodic table and the symbol is ‘br’. In this article today we are going to tell you about the electron configuration of bromine, its orbital. 1 determine. Bromine Has 28 Core Electrons.