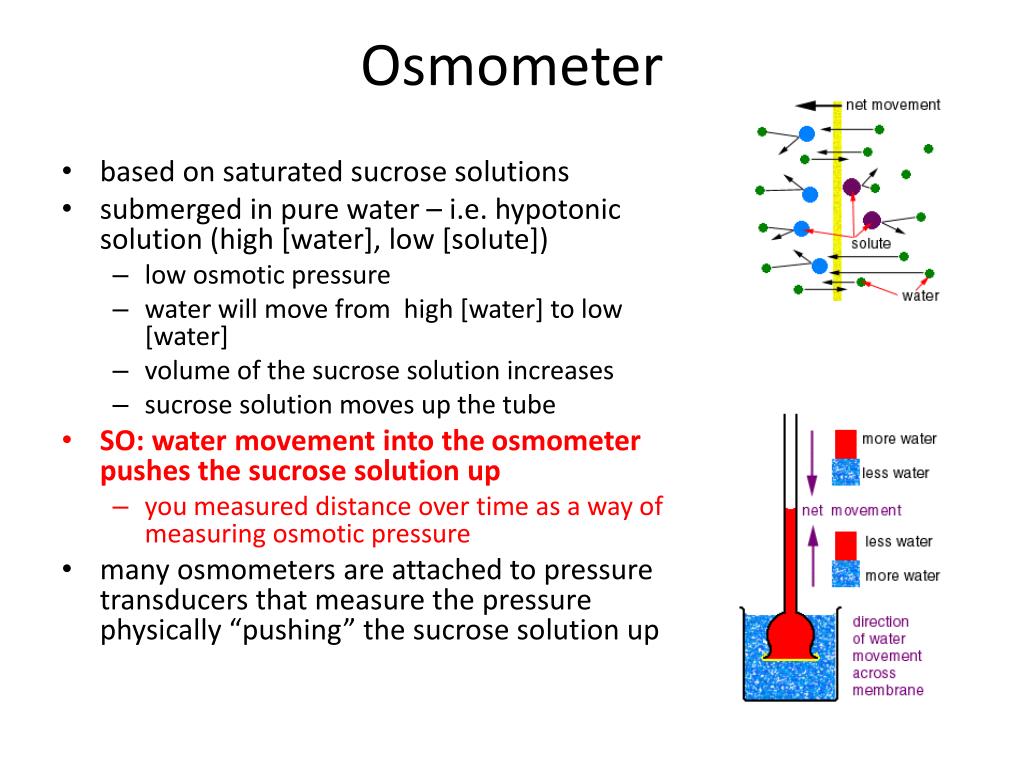
Osmometer Meaning In Anatomy . Osmolarity of a solution is the number of osmoles of solute per. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. Most modern osmometers share a common principle: An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. In this lesson, we will learn how it is typically measured as well as the pros of the most. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. Osmolality is often used to determine dehydration. Types of osmometers and their operating principle.
from www.slideserve.com
Osmolality is often used to determine dehydration. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. Most modern osmometers share a common principle: Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolarity of a solution is the number of osmoles of solute per. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. In this lesson, we will learn how it is typically measured as well as the pros of the most. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Types of osmometers and their operating principle.
PPT Labs 6 & 7 PowerPoint Presentation, free download ID2180146
Osmometer Meaning In Anatomy An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Osmolality is often used to determine dehydration. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. Osmolarity of a solution is the number of osmoles of solute per. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Types of osmometers and their operating principle. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. In this lesson, we will learn how it is typically measured as well as the pros of the most. Most modern osmometers share a common principle:
From www.biophlox.com
A Complete Lab Guide to Osmometer Osmometer Meaning In Anatomy Osmolarity of a solution is the number of osmoles of solute per. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. Most modern osmometers share a common principle: Osmolality. Osmometer Meaning In Anatomy.
From www.alamy.com
Osmometer hires stock photography and images Alamy Osmometer Meaning In Anatomy The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. Most modern osmometers share a common principle: Osmolality of a solution is the number of osmoles of solute per kilogram. Osmometer Meaning In Anatomy.
From www.researchgate.net
Schematic diagram of burial of typical osmometer (DBCP35) and cable... Download Scientific Osmometer Meaning In Anatomy Osmolality is often used to determine dehydration. Most modern osmometers share a common principle: Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Types of osmometers and their operating principle. In. Osmometer Meaning In Anatomy.
From www.aquaportail.com
Osmomètre définition et explications Osmometer Meaning In Anatomy Types of osmometers and their operating principle. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. In this lesson, we will learn how it is typically measured as well as the pros of the most. Osmolality is often used to determine dehydration. Osmolarity of a solution. Osmometer Meaning In Anatomy.
From www.youtube.com
Potato Osmometer Experiment Class11 / Transport in Plants/ BIOLOGY Wings Bhagalpur YouTube Osmometer Meaning In Anatomy Osmolarity of a solution is the number of osmoles of solute per. Most modern osmometers share a common principle: Types of osmometers and their operating principle. Osmolality is often used to determine dehydration. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. In this lesson, we. Osmometer Meaning In Anatomy.
From www.researchgate.net
Osmometers for measurement of colloid osmotic pressure inside cells.... Download Scientific Osmometer Meaning In Anatomy An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. In this lesson, we will learn how it is typically measured as well as the pros of the most. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments. Osmometer Meaning In Anatomy.
From faculty.csbsju.edu
Plant Physiology Osmometer Meaning In Anatomy In this lesson, we will learn how it is typically measured as well as the pros of the most. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments. Osmometer Meaning In Anatomy.
From www.camlab.co.uk
Introducing Two New Osmometers Camlab Osmometer Meaning In Anatomy There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. In this lesson, we will learn how it is typically measured as well as the pros of the most. Types of osmometers and their operating principle. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps. Osmometer Meaning In Anatomy.
From www.il-biosystems.com
OsmoPRO MAX Osmometer Efficient Osmolality Determination Osmometer Meaning In Anatomy Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. Osmolality is often used to determine dehydration. Osmolarity of a solution is the number of osmoles of solute per. The maintenance of adequate body fluid volume and the. Osmometer Meaning In Anatomy.
From www.slideserve.com
PPT Clinical Chemistry Chapter 4 PowerPoint Presentation, free download ID1296071 Osmometer Meaning In Anatomy In this lesson, we will learn how it is typically measured as well as the pros of the most. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. Osmolarity of a solution is the number of osmoles of solute per. Types of osmometers and their operating. Osmometer Meaning In Anatomy.
From wellcomecollection.org
Diagram of Graham's Osmometer (to study osmosis). Collection Osmometer Meaning In Anatomy The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Osmolality of a solution is the number of osmoles of solute per kilogram of. Osmometer Meaning In Anatomy.
From studylib.net
Membrane Osmometry ( , A , χ) M 2 Osmometer Meaning In Anatomy Osmolality is often used to determine dehydration. Osmolarity of a solution is the number of osmoles of solute per. In this lesson, we will learn how it is typically measured as well as the pros of the most. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Most modern osmometers share a common principle:. Osmometer Meaning In Anatomy.
From www.youtube.com
Osmometer Meaning YouTube Osmometer Meaning In Anatomy There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. Most modern osmometers share a common principle: Osmolarity of a solution is the number of osmoles of solute per. In. Osmometer Meaning In Anatomy.
From slidetodoc.com
Membrane Osmometry Alfredo Clemente CH 392 N Prof Osmometer Meaning In Anatomy An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Osmolality is often used to determine dehydration. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. Most modern osmometers share a common principle:. Osmometer Meaning In Anatomy.
From www.mbm-lehrmittel.de
Osmometer Osmose Physiologie Biologie MBM Lehrmittel & Verlagsgesellschaft mbH Osmometer Meaning In Anatomy In this lesson, we will learn how it is typically measured as well as the pros of the most. Osmolality is often used to determine dehydration. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is. Osmometer Meaning In Anatomy.
From www.iconsci.com
What Are Osmometers? Osmometer Meaning In Anatomy The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Types of osmometers and their operating principle. Osmolality is often used to determine dehydration.. Osmometer Meaning In Anatomy.
From www.flickr.com
Membrane Osmometry Diagram of a membrane osmometer. Format… Flickr Osmometer Meaning In Anatomy Osmolarity of a solution is the number of osmoles of solute per. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. In this lesson, we will learn how it is typically measured as well as the pros of the most. Osmolality of a solution is the. Osmometer Meaning In Anatomy.
From www.researchgate.net
The osmometer properties of swelling protoplasts. A, a, An... Download Scientific Diagram Osmometer Meaning In Anatomy In this lesson, we will learn how it is typically measured as well as the pros of the most. Types of osmometers and their operating principle. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body. Osmometer Meaning In Anatomy.
From www.slideserve.com
PPT Diffusion and Osmosis PowerPoint Presentation, free download ID4917478 Osmometer Meaning In Anatomy Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. Types of osmometers and their. Osmometer Meaning In Anatomy.
From www.researchgate.net
Types of osmometers. Download Scientific Diagram Osmometer Meaning In Anatomy There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. Osmolarity of a solution is the number of osmoles of solute per. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. The maintenance of adequate body fluid volume and. Osmometer Meaning In Anatomy.
From dir.indiamart.com
Osmometer at Best Price in India Osmometer Meaning In Anatomy Osmolality is often used to determine dehydration. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. There are two principal methods of osmometry. Osmometer Meaning In Anatomy.
From www.chegg.com
Solved 2. The experimental setup below shows an osmometer. Osmometer Meaning In Anatomy An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. In this lesson, we will learn how it is typically measured as well as the pros of the most. Osmolarity of a solution is the number of osmoles of solute per. Types of osmometers and their operating. Osmometer Meaning In Anatomy.
From shop-ntl.eu
Osmometer Osmometer Meaning In Anatomy There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Osmolarity of a solution is the number of osmoles of solute per. The maintenance of adequate body fluid volume and. Osmometer Meaning In Anatomy.
From slidetodoc.com
Membrane Osmometry Alfredo Clemente CH 392 N Prof Osmometer Meaning In Anatomy There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. In this lesson, we will learn how it is typically measured as well as the pros of the most. Most modern osmometers share a common principle: An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps. Osmometer Meaning In Anatomy.
From www.scribd.com
osmometer Osmometer Meaning In Anatomy There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. In this lesson, we will learn how it is typically measured as well as the pros of the most. Types of osmometers and their operating principle. Osmolality is. Osmometer Meaning In Anatomy.
From www.slideserve.com
PPT Diffusion and Osmosis PowerPoint Presentation, free download ID4917478 Osmometer Meaning In Anatomy There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. Types of osmometers and their operating principle. An osmometer is a scientific instrument used to measure the osmotic pressure of. Osmometer Meaning In Anatomy.
From hylandscientific.com
Advanced Instruments Model Osmo1 Osmometer with Accessories Hyland Scientific Osmometer Meaning In Anatomy Types of osmometers and their operating principle. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Osmolality is often used to determine dehydration. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolarity of a solution is the number of. Osmometer Meaning In Anatomy.
From www.slideserve.com
PPT Labs 6 & 7 PowerPoint Presentation, free download ID2180146 Osmometer Meaning In Anatomy Osmolality is often used to determine dehydration. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Types of osmometers and their operating principle. Most modern osmometers share a common principle: Osmolarity of a solution is the number of osmoles of solute per. Osmolality of a solution. Osmometer Meaning In Anatomy.
From www.slideserve.com
PPT Molecular Weight and polymer properties Methods Used to determine Mn, Mw Membrane Osmometer Meaning In Anatomy An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. Osmolarity of a solution is the number of osmoles of solute per. In this. Osmometer Meaning In Anatomy.
From www.goconqr.com
Movement of Substances into and out of Cells(2Pages) Note Osmometer Meaning In Anatomy There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Most modern osmometers share a. Osmometer Meaning In Anatomy.
From www.scribd.com
Practical 5. Study of Osmosis by Potato Osmometer PDF Osmometer Meaning In Anatomy An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part. Osmometer Meaning In Anatomy.
From www.mrclab.com
Osmometer Instrument Osmometer Meaning In Anatomy An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. Osmolality is often used to determine dehydration. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a critical part of. There are two principal methods of osmometry. Osmometer Meaning In Anatomy.
From www.scribd.com
osmometry ppt Osmometer Meaning In Anatomy An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:. The maintenance of adequate body fluid volume and the correct distribution of this fluid between the body compartments is a. Osmometer Meaning In Anatomy.
From www.youtube.com
Osmotic Pressure Osmosis Colligative property Physiology Series YouTube Osmometer Meaning In Anatomy Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolality is often used to determine dehydration. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. The maintenance of adequate body fluid volume and the correct distribution of this fluid between. Osmometer Meaning In Anatomy.
From testbook.com
Understanding Osmosis Using Potato Osmometer Osmometer Meaning In Anatomy Osmolality is often used to determine dehydration. In this lesson, we will learn how it is typically measured as well as the pros of the most. An osmometer is a scientific instrument used to measure the osmotic pressure of a solution, which helps to determine the concentration of solutes. The maintenance of adequate body fluid volume and the correct distribution. Osmometer Meaning In Anatomy.