Acetic Acid Conjugate Base . Learn about its structure, properties, preparation. evaluate k a of the conjugate acid of a base. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Once again, we have two conjugate. Treat the conjugate acid of a base as an acid in numerical calculations. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. There are two acids and.
from www.chegg.com
whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. Learn about its structure, properties, preparation. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. evaluate k a of the conjugate acid of a base. There are two acids and. Treat the conjugate acid of a base as an acid in numerical calculations. Once again, we have two conjugate. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization.
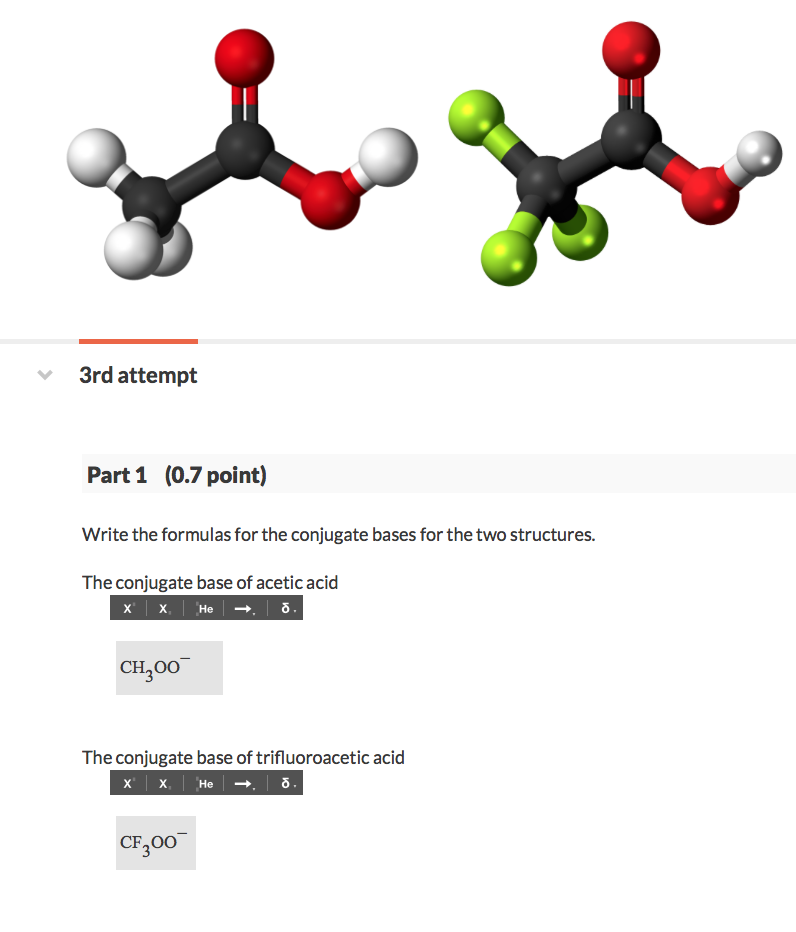
Solved Acetic acid (CH3COOH) and trifluoroacetic acid
Acetic Acid Conjugate Base learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Treat the conjugate acid of a base as an acid in numerical calculations. Once again, we have two conjugate. Learn about its structure, properties, preparation. evaluate k a of the conjugate acid of a base. There are two acids and. whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization.
From
Acetic Acid Conjugate Base evaluate k a of the conjugate acid of a base. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. There are two acids and. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. Treat the conjugate acid of a. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base Once again, we have two conjugate. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. learn how to identify conjugate acids and bases, and how they. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. evaluate k a of the conjugate acid of a base. acetic acid / əˈsiːtɪk /, systematically named ethanoic. Acetic Acid Conjugate Base.
From www.slideserve.com
PPT Conjugate Acidbase pairs PowerPoint Presentation, free download Acetic Acid Conjugate Base There are two acids and. evaluate k a of the conjugate acid of a base. Once again, we have two conjugate. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. learn how to identify conjugate acids and bases, and how they relate to acid strength. Acetic Acid Conjugate Base.
From exolobhyy.blob.core.windows.net
Acetic Acid Xylene Azeotrope at Donna Delisle blog Acetic Acid Conjugate Base Learn about its structure, properties, preparation. evaluate k a of the conjugate acid of a base. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. whenever an acid donates. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base Treat the conjugate acid of a base as an acid in numerical calculations. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. Learn about its structure, properties, preparation. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and. Acetic Acid Conjugate Base.
From www.numerade.com
SOLVED You need to produce a buffer solution that has a pH of 5.49 Acetic Acid Conjugate Base Treat the conjugate acid of a base as an acid in numerical calculations. There are two acids and. Once again, we have two conjugate. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base Treat the conjugate acid of a base as an acid in numerical calculations. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Learn about its structure, properties, preparation. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. evaluate k. Acetic Acid Conjugate Base.
From www.slideserve.com
PPT Equilibrium Acids and Bases PowerPoint Presentation, free Acetic Acid Conjugate Base learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. There are two acids and. evaluate k a of the conjugate acid of a base. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Treat the conjugate acid of a. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. There are two acids and. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Once again, we have two conjugate. whenever an acid donates a proton,. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base There are two acids and. Learn about its structure, properties, preparation. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. in the reverse reaction, \(h_3o^+\) is the acid that donates. Acetic Acid Conjugate Base.
From learningfullmanteau.z5.web.core.windows.net
Conjugate Acids And Bases Worksheets Acetic Acid Conjugate Base There are two acids and. Learn about its structure, properties, preparation. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Treat the conjugate acid of a base as an acid in numerical calculations. learn how to identify conjugate acids and bases, and how they relate to acid strength. Acetic Acid Conjugate Base.
From ar.inspiredpencil.com
Acid Base Reaction Examples Acetic Acid Conjugate Base acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. Learn about its structure, properties, preparation. Once again, we have two conjugate. Treat the conjugate acid of a. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton,. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base Learn about its structure, properties, preparation. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Once again, we have two conjugate. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. evaluate k a of the. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base There are two acids and. evaluate k a of the conjugate acid of a base. whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. in the reverse. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. evaluate k a of the conjugate acid of a base. Once again, we have two conjugate. whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. Treat the conjugate. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. There are two acids and. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. evaluate k a of the conjugate acid of a base. Treat the. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Treat the conjugate acid of a base as an acid in numerical calculations. in the reverse reaction,. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. Learn about its structure, properties, preparation. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. evaluate k a of the conjugate acid of a base. . Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. Treat the conjugate acid of a base as an acid in numerical calculations. Once again, we have two conjugate. Learn about its structure, properties, preparation. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base Treat the conjugate acid of a base as an acid in numerical calculations. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. acetic acid / əˈsiːtɪk /, systematically. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base Learn about its structure, properties, preparation. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Treat the conjugate acid of a base as an acid in numerical calculations. Once again, we. Acetic Acid Conjugate Base.
From slideplayer.com
The Nature of AcidBase Equilibria ppt download Acetic Acid Conjugate Base Treat the conjugate acid of a base as an acid in numerical calculations. Once again, we have two conjugate. evaluate k a of the conjugate acid of a base. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. whenever an acid donates a proton, the. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base There are two acids and. Learn about its structure, properties, preparation. whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. Treat the conjugate acid of a base as an acid in numerical calculations. learn how to identify conjugate acids and bases, and how they relate to. Acetic Acid Conjugate Base.
From slideplayer.com
Weak Acids and Bases. ppt download Acetic Acid Conjugate Base Treat the conjugate acid of a base as an acid in numerical calculations. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. Once again, we have two conjugate. . Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base Learn about its structure, properties, preparation. evaluate k a of the conjugate acid of a base. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. Treat. Acetic Acid Conjugate Base.
From www.chegg.com
Solved 12. Draw the conjugate base of acetic acid with its Acetic Acid Conjugate Base in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization.. Acetic Acid Conjugate Base.
From slideplayer.com
Chapter 8 Acids and Bases ppt download Acetic Acid Conjugate Base Treat the conjugate acid of a base as an acid in numerical calculations. There are two acids and. Once again, we have two conjugate. evaluate k a of the conjugate acid of a base. Learn about its structure, properties, preparation. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. . Acetic Acid Conjugate Base.
From slideplayer.com
EQUILBRIUM OF Acids and Bases ppt download Acetic Acid Conjugate Base evaluate k a of the conjugate acid of a base. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion, which acts as the base. Learn about its structure, properties, preparation. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. . Acetic Acid Conjugate Base.
From www.slideserve.com
PPT Acids, Bases and pH PowerPoint Presentation, free download ID Acetic Acid Conjugate Base evaluate k a of the conjugate acid of a base. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Learn about its structure, properties, preparation. Once again, we have two conjugate. in the reverse reaction, \(h_3o^+\) is the acid that donates a proton to the acetate ion,. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. Once again, we have two conjugate. There are two acids and. evaluate k a of the conjugate acid of a base. Learn about its structure, properties, preparation. Treat the conjugate acid of a base as an acid. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base Once again, we have two conjugate. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. There are two acids and. learn how to identify conjugate acids and bases, and how they relate to acid strength and ionization. in the reverse reaction, \(h_3o^+\) is the acid that donates. Acetic Acid Conjugate Base.
From
Acetic Acid Conjugate Base whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid. evaluate k a of the conjugate acid of a base. acetic acid / əˈsiːtɪk /, systematically named ethanoic acid / ˌɛθəˈnoʊɪk /, is an acidic, colourless liquid and organic. Learn about its structure, properties, preparation. Once. Acetic Acid Conjugate Base.