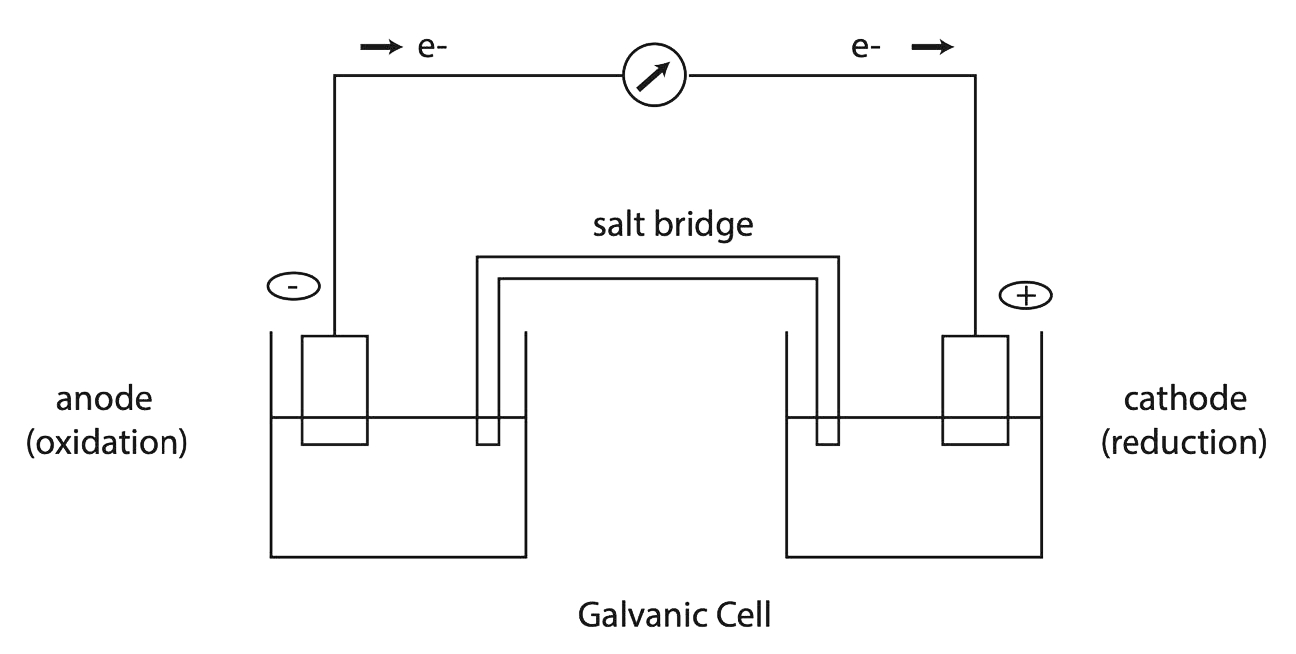
Use Of Electrochemical Cell In Daily Life . Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. The hydrogen fuel cell uses hydrogen and oxygen. What is an electrochemical cell? An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or.
from chem.libretexts.org
An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. The hydrogen fuel cell uses hydrogen and oxygen. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. What is an electrochemical cell?
1 Electrochemical Cells (Experiment) Chemistry LibreTexts
Use Of Electrochemical Cell In Daily Life What is an electrochemical cell? The hydrogen fuel cell uses hydrogen and oxygen. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. What is an electrochemical cell? Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets.
From en.ppt-online.org
Electrochemistry online presentation Use Of Electrochemical Cell In Daily Life Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons. Use Of Electrochemical Cell In Daily Life.
From www.alamy.com
Educational Diagram of Chart showing Physics concept of Electrochemical Use Of Electrochemical Cell In Daily Life Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. An electrochemical. Use Of Electrochemical Cell In Daily Life.
From www.vectorstock.com
Electrochemical cell or galvanic daniell Vector Image Use Of Electrochemical Cell In Daily Life The hydrogen fuel cell uses hydrogen and oxygen. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with. Use Of Electrochemical Cell In Daily Life.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Use Of Electrochemical Cell In Daily Life An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy. Use Of Electrochemical Cell In Daily Life.
From edu-rsc-org-ssl.oca.korea.ac.kr
Teach electrochemical cells using number grids Ideas RSC Education Use Of Electrochemical Cell In Daily Life Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Electrochemistry seems a little. Use Of Electrochemical Cell In Daily Life.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples Use Of Electrochemical Cell In Daily Life The hydrogen fuel cell uses hydrogen and oxygen. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. What is an electrochemical cell? Electrochemistry seems a little. Use Of Electrochemical Cell In Daily Life.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Use Of Electrochemical Cell In Daily Life Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and. Use Of Electrochemical Cell In Daily Life.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts Use Of Electrochemical Cell In Daily Life An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The hydrogen fuel cell uses hydrogen and oxygen. Electrochemical cells are devices for turning chemical energy into electrical energy. Use Of Electrochemical Cell In Daily Life.
From mantavya.com
What Is Electroplating & How does it work 2021 Guide Mantavya Use Of Electrochemical Cell In Daily Life An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. The hydrogen fuel cell uses hydrogen and oxygen. Electrochemistry seems a little intimidating at first, but we explain. Use Of Electrochemical Cell In Daily Life.
From mungfali.com
Galvanic Cell Example Use Of Electrochemical Cell In Daily Life An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs. Use Of Electrochemical Cell In Daily Life.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Use Of Electrochemical Cell In Daily Life An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. What is an electrochemical cell? Fuel cells are similar to batteries in that they generate an electrical. Use Of Electrochemical Cell In Daily Life.
From ar.inspiredpencil.com
Electrolytic Cell Use Of Electrochemical Cell In Daily Life Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. What is an electrochemical cell? An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. The hydrogen fuel cell uses hydrogen and oxygen. Electrochemistry seems a little intimidating at first, but. Use Of Electrochemical Cell In Daily Life.
From www.youtube.com
ELECTROCHEMICAL CELL HINDI EXPLANATION ELECTROCHEMISTRY 12TH Use Of Electrochemical Cell In Daily Life An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Electrochemistry seems a little intimidating at first, but we explain some of. Use Of Electrochemical Cell In Daily Life.
From www.vrogue.co
Examples Of Galvanic Cells In Everyday Life Studiousg vrogue.co Use Of Electrochemical Cell In Daily Life The hydrogen fuel cell uses hydrogen and oxygen. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. What is an electrochemical cell? An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Electrochemical cells are. Use Of Electrochemical Cell In Daily Life.
From 2012books.lardbucket.org
Describing Electrochemical Cells Use Of Electrochemical Cell In Daily Life An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. An electrochemical cell is a device capable. Use Of Electrochemical Cell In Daily Life.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Use Of Electrochemical Cell In Daily Life Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations. Use Of Electrochemical Cell In Daily Life.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Use Of Electrochemical Cell In Daily Life Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. What is an electrochemical cell? The hydrogen fuel cell uses hydrogen and oxygen. An electrochemical. Use Of Electrochemical Cell In Daily Life.
From dokumen.tips
(PPT) Electrochemical & Electrolytic Cells Using Redox Reactions in Use Of Electrochemical Cell In Daily Life What is an electrochemical cell? Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. An electrochemical cell splits the oxidant and reductant in. Use Of Electrochemical Cell In Daily Life.
From mavink.com
Diagram Of Electrochemical Cell Use Of Electrochemical Cell In Daily Life Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Fuel cells are similar to batteries in that they. Use Of Electrochemical Cell In Daily Life.
From www.youtube.com
Uses of Electrochemistry in our daily life (Electrochemistry part 2 for Use Of Electrochemical Cell In Daily Life An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. Electrochemical cells are devices for turning chemical. Use Of Electrochemical Cell In Daily Life.
From www.vrogue.co
Electrochemical Cell Or Galvanic Cell The Daniell Cel vrogue.co Use Of Electrochemical Cell In Daily Life Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. Electrochemical cells are devices for turning chemical energy into electrical energy. Use Of Electrochemical Cell In Daily Life.
From facts.net
15 Surprising Facts About Electrochemical Cell Use Of Electrochemical Cell In Daily Life What is an electrochemical cell? Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through. Use Of Electrochemical Cell In Daily Life.
From pandai.me
Electrolytic Cell Use Of Electrochemical Cell In Daily Life An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow. Use Of Electrochemical Cell In Daily Life.
From www.thoughtco.com
Electrochemical Cell Definition Use Of Electrochemical Cell In Daily Life An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing. Use Of Electrochemical Cell In Daily Life.
From 2012books.lardbucket.org
Electrochemistry Use Of Electrochemical Cell In Daily Life An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. The hydrogen fuel cell uses hydrogen and oxygen. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively,. Use Of Electrochemical Cell In Daily Life.
From brainly.com
I NEED HELP PLEASE, THANKS! Electrochemistry is important in many Use Of Electrochemical Cell In Daily Life Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. What is an electrochemical cell? An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. Fuel cells are similar to batteries in that they generate an electrical current,. Use Of Electrochemical Cell In Daily Life.
From www.firsthope.co.in
Electrochemical cell Use Of Electrochemical Cell In Daily Life Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. The hydrogen fuel cell uses hydrogen and oxygen. An electrochemical cell splits the oxidant and. Use Of Electrochemical Cell In Daily Life.
From saylordotorg.github.io
Describing Electrochemical Cells Use Of Electrochemical Cell In Daily Life An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. The hydrogen fuel cell uses hydrogen and oxygen. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. Electrochemical cells are devices. Use Of Electrochemical Cell In Daily Life.
From hxeljcwuc.blob.core.windows.net
Electrochemical Cell Real Life Examples at Steven Smith blog Use Of Electrochemical Cell In Daily Life What is an electrochemical cell? The hydrogen fuel cell uses hydrogen and oxygen. Electrochemical cells are devices for turning chemical energy into electrical energy or, alternatively, changing electrical energy into. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. An electrochemical cell produces an. Use Of Electrochemical Cell In Daily Life.
From www.slideserve.com
PPT Electrolytic Cell PowerPoint Presentation, free download ID2809508 Use Of Electrochemical Cell In Daily Life An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. The hydrogen fuel cell uses hydrogen and oxygen. Electrochemical cells are devices for turning. Use Of Electrochemical Cell In Daily Life.
From www.vecteezy.com
Electrochemical cell or Galvanic cell, The Daniell cell 18989226 Vector Use Of Electrochemical Cell In Daily Life An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. The hydrogen fuel cell uses hydrogen and oxygen. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. What is an electrochemical. Use Of Electrochemical Cell In Daily Life.
From www.vrogue.co
Examples Of Galvanic Cells In Everyday Life Studiousg vrogue.co Use Of Electrochemical Cell In Daily Life What is an electrochemical cell? An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. The hydrogen fuel cell uses hydrogen and oxygen. Electrochemical. Use Of Electrochemical Cell In Daily Life.
From www.vrogue.co
Examples Of Galvanic Cells In Everyday Life Studiousg vrogue.co Use Of Electrochemical Cell In Daily Life An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to flow through an external circuit from the reductant (which gets. An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos,. Use Of Electrochemical Cell In Daily Life.
From www.youtube.com
Electrochemistry Classification Type Of Cell Electrochemical Use Of Electrochemical Cell In Daily Life Fuel cells are similar to batteries in that they generate an electrical current, but require continuous addition of fuel and oxidizer. The hydrogen fuel cell uses hydrogen and oxygen. An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. What is an electrochemical cell? An electrochemical cell. Use Of Electrochemical Cell In Daily Life.
From www.slideserve.com
PPT Batteries and Fuel Cells PowerPoint Presentation, free download Use Of Electrochemical Cell In Daily Life An electrochemical cell produces an electric current through chemical reactions, or it can do the opposite, producing or. What is an electrochemical cell? Electrochemistry seems a little intimidating at first, but we explain some of the concepts here with videos, gifs and explanations and show you how it’s relevant with examples. Fuel cells are similar to batteries in that they. Use Of Electrochemical Cell In Daily Life.