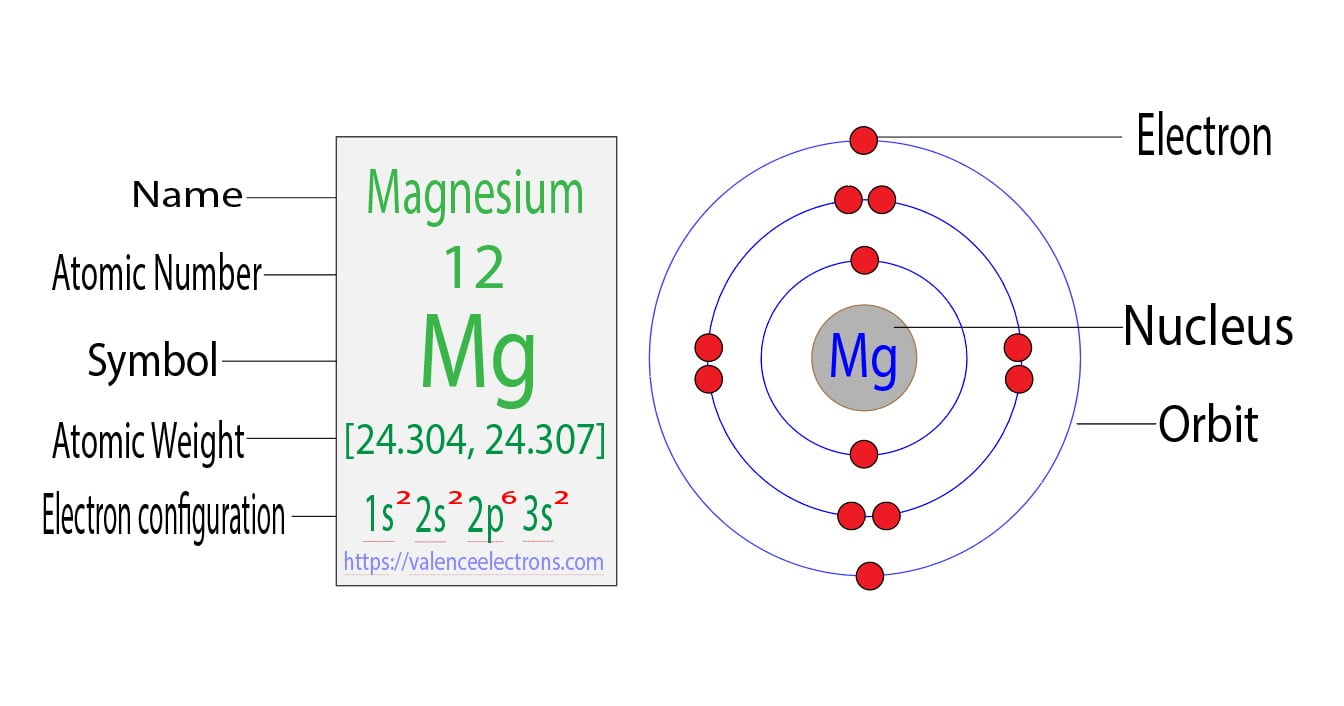
Magnesium Electron Rings . The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Each entry has a full citation identifying its source. Electron configuration of magnesium is [ne] 3s2. Magnesium occurs naturally only in. The formula of the compound is mgo. You can see in the dot structure that the two atoms share four different electrons. Magnesium (mg) is able to bond with one oxygen (o) atom. Our magnesium page has over 360 facts that span 117 different quantities. Since 1s can only hold two electrons the. The electron shells are shown, moving outward from the nucleus. Possible oxidation states are +2.
from valenceelectrons.com
Magnesium (mg) is able to bond with one oxygen (o) atom. The formula of the compound is mgo. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Each entry has a full citation identifying its source. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Since 1s can only hold two electrons the. You can see in the dot structure that the two atoms share four different electrons. Possible oxidation states are +2. Electron configuration of magnesium is [ne] 3s2. The electron shells are shown, moving outward from the nucleus.
How to Write the Electron Configuration for Magnesium (Mg)?
Magnesium Electron Rings Magnesium (mg) is able to bond with one oxygen (o) atom. Our magnesium page has over 360 facts that span 117 different quantities. Since 1s can only hold two electrons the. The formula of the compound is mgo. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. You can see in the dot structure that the two atoms share four different electrons. Magnesium (mg) is able to bond with one oxygen (o) atom. Each entry has a full citation identifying its source. Possible oxidation states are +2. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Electron configuration of magnesium is [ne] 3s2. Magnesium occurs naturally only in. The electron shells are shown, moving outward from the nucleus.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Electron Rings Each entry has a full citation identifying its source. Since 1s can only hold two electrons the. Possible oxidation states are +2. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The formula of the compound is mgo. You can see in the dot structure that the two atoms share four different. Magnesium Electron Rings.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Electron Rings The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The formula of the compound is mgo. Each entry has a full citation identifying its source. You can see in the dot structure that the two atoms share four different electrons. Since 1s can only hold two electrons the. Possible. Magnesium Electron Rings.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Magnesium Electron Rings In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Electron configuration of magnesium is [ne] 3s2. Our magnesium page has over 360 facts that span 117 different quantities. You can see in the dot structure that the two atoms share four different electrons. The formula of the compound is mgo. The electron. Magnesium Electron Rings.
From www.teachoo.com
(i) Write the electrondot structures for sodium, oxygen and magnesium Magnesium Electron Rings The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Electron configuration of magnesium is [ne] 3s2. Possible oxidation states are +2. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. You can see in the dot structure that the two atoms. Magnesium Electron Rings.
From slidetodoc.com
Warm Up 1 Draw the electron configuration rings Magnesium Electron Rings Magnesium occurs naturally only in. Our magnesium page has over 360 facts that span 117 different quantities. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Each entry has a full citation identifying its source. Magnesium (mg) is able to bond with one oxygen (o) atom. In writing the. Magnesium Electron Rings.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Electron Rings Electron configuration of magnesium is [ne] 3s2. Magnesium (mg) is able to bond with one oxygen (o) atom. Magnesium occurs naturally only in. The formula of the compound is mgo. The electron shells are shown, moving outward from the nucleus. Each entry has a full citation identifying its source. The final ring or shell of electrons contains the typical number. Magnesium Electron Rings.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Rings Magnesium (mg) is able to bond with one oxygen (o) atom. The formula of the compound is mgo. You can see in the dot structure that the two atoms share four different electrons. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Each entry has a full citation identifying. Magnesium Electron Rings.
From www.animalia-life.club
Electron Shell Diagram Magnesium Electron Rings The formula of the compound is mgo. You can see in the dot structure that the two atoms share four different electrons. The electron shells are shown, moving outward from the nucleus. Magnesium (mg) is able to bond with one oxygen (o) atom. Possible oxidation states are +2. Magnesium occurs naturally only in. Electron configuration of magnesium is [ne] 3s2.. Magnesium Electron Rings.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Electron Rings Magnesium (mg) is able to bond with one oxygen (o) atom. Magnesium occurs naturally only in. Our magnesium page has over 360 facts that span 117 different quantities. The electron shells are shown, moving outward from the nucleus. Since 1s can only hold two electrons the. Each entry has a full citation identifying its source. The final ring or shell. Magnesium Electron Rings.
From www.shutterstock.com
Bohr Model Magnesium Atom Electron Structure เวกเตอร์สต็อก (ปลอดค่า Magnesium Electron Rings The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Magnesium occurs naturally only in. Possible oxidation states are +2. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The electron shells are shown, moving outward from the nucleus. You can see. Magnesium Electron Rings.
From commons.wikimedia.org
FileElectron shell 012 Magnesium.svg Wikimedia Commons Magnesium Electron Rings You can see in the dot structure that the two atoms share four different electrons. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium (mg) is able to bond with one oxygen (o) atom. Since 1s can only hold two electrons the. Our magnesium page has over 360 facts that span. Magnesium Electron Rings.
From www.nagwa.com
Question Video Identifying the Element of an Atom from its Electron Magnesium Electron Rings Each entry has a full citation identifying its source. Possible oxidation states are +2. Since 1s can only hold two electrons the. Our magnesium page has over 360 facts that span 117 different quantities. The formula of the compound is mgo. Magnesium (mg) is able to bond with one oxygen (o) atom. The final ring or shell of electrons contains. Magnesium Electron Rings.
From www.youtube.com
Magnesium Electron Configuration YouTube Magnesium Electron Rings You can see in the dot structure that the two atoms share four different electrons. Electron configuration of magnesium is [ne] 3s2. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The electron shells are shown, moving outward from the nucleus. Our magnesium page has over 360 facts that. Magnesium Electron Rings.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Rings Magnesium occurs naturally only in. Magnesium (mg) is able to bond with one oxygen (o) atom. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The formula of the compound is mgo. Since 1s can only hold two electrons the. Our magnesium page has over 360 facts that span 117 different quantities.. Magnesium Electron Rings.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Magnesium Electron Rings The electron shells are shown, moving outward from the nucleus. You can see in the dot structure that the two atoms share four different electrons. Since 1s can only hold two electrons the. Magnesium (mg) is able to bond with one oxygen (o) atom. Our magnesium page has over 360 facts that span 117 different quantities. Each entry has a. Magnesium Electron Rings.
From www.dreamstime.com
Electron of the Element Magnesium Stock Vector Illustration of Magnesium Electron Rings Possible oxidation states are +2. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Electron configuration of magnesium is [ne] 3s2. The formula of the compound is mgo. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Each entry has a. Magnesium Electron Rings.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Rings The formula of the compound is mgo. Since 1s can only hold two electrons the. Magnesium (mg) is able to bond with one oxygen (o) atom. The electron shells are shown, moving outward from the nucleus. You can see in the dot structure that the two atoms share four different electrons. Electron configuration of magnesium is [ne] 3s2. In writing. Magnesium Electron Rings.
From www.expii.com
Valence Electrons — Definition & Importance Expii Magnesium Electron Rings You can see in the dot structure that the two atoms share four different electrons. Magnesium occurs naturally only in. Since 1s can only hold two electrons the. Possible oxidation states are +2. Magnesium (mg) is able to bond with one oxygen (o) atom. Our magnesium page has over 360 facts that span 117 different quantities. In writing the electron. Magnesium Electron Rings.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Electron Rings Possible oxidation states are +2. Our magnesium page has over 360 facts that span 117 different quantities. Magnesium (mg) is able to bond with one oxygen (o) atom. Each entry has a full citation identifying its source. Magnesium occurs naturally only in. The electron shells are shown, moving outward from the nucleus. Electron configuration of magnesium is [ne] 3s2. Since. Magnesium Electron Rings.
From www.sciencephoto.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Library Magnesium Electron Rings The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Electron configuration of magnesium is [ne] 3s2. Each entry has a full citation identifying its source. The formula of the compound is mgo. Magnesium occurs naturally only in. In writing the electron configuration for magnesium the first two electrons will. Magnesium Electron Rings.
From nursehub.com
Electron Shells NurseHub Magnesium Electron Rings The formula of the compound is mgo. You can see in the dot structure that the two atoms share four different electrons. Possible oxidation states are +2. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Electron configuration of magnesium is [ne] 3s2. Since 1s can only hold two electrons the. The. Magnesium Electron Rings.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Electron Rings Possible oxidation states are +2. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the. Each entry has a full citation identifying its source. Our magnesium page has over 360 facts that span 117 different quantities. The formula of the compound is mgo. The electron. Magnesium Electron Rings.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Rings Each entry has a full citation identifying its source. Possible oxidation states are +2. The formula of the compound is mgo. You can see in the dot structure that the two atoms share four different electrons. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Electron configuration of magnesium is [ne] 3s2.. Magnesium Electron Rings.
From www.toppr.com
Write the electron dot structure of magnesium and oxygen. Magnesium Electron Rings Possible oxidation states are +2. The electron shells are shown, moving outward from the nucleus. Our magnesium page has over 360 facts that span 117 different quantities. Electron configuration of magnesium is [ne] 3s2. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium occurs naturally only in. The final ring or. Magnesium Electron Rings.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Electron Rings Since 1s can only hold two electrons the. Magnesium (mg) is able to bond with one oxygen (o) atom. The electron shells are shown, moving outward from the nucleus. The formula of the compound is mgo. Electron configuration of magnesium is [ne] 3s2. You can see in the dot structure that the two atoms share four different electrons. In writing. Magnesium Electron Rings.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Electron Rings In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Electron configuration of magnesium is [ne] 3s2. You can see in the dot structure that the two atoms share four different electrons. Possible oxidation states are +2. Magnesium occurs naturally only in. Since 1s can only hold two electrons the. Magnesium (mg) is. Magnesium Electron Rings.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Magnesium Electron Rings The electron shells are shown, moving outward from the nucleus. Each entry has a full citation identifying its source. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Since 1s can only hold two electrons the. The formula of the compound is mgo. Possible oxidation states are +2. Magnesium. Magnesium Electron Rings.
From www.slideserve.com
PPT Isotopes of Magnesium PowerPoint Presentation, free download ID Magnesium Electron Rings Each entry has a full citation identifying its source. Our magnesium page has over 360 facts that span 117 different quantities. Magnesium occurs naturally only in. Possible oxidation states are +2. You can see in the dot structure that the two atoms share four different electrons. The final ring or shell of electrons contains the typical number of valence electrons. Magnesium Electron Rings.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Electron Rings Magnesium occurs naturally only in. Possible oxidation states are +2. Electron configuration of magnesium is [ne] 3s2. Since 1s can only hold two electrons the. The formula of the compound is mgo. Magnesium (mg) is able to bond with one oxygen (o) atom. Each entry has a full citation identifying its source. The electron shells are shown, moving outward from. Magnesium Electron Rings.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Electron Rings Electron configuration of magnesium is [ne] 3s2. Each entry has a full citation identifying its source. Magnesium occurs naturally only in. The electron shells are shown, moving outward from the nucleus. Magnesium (mg) is able to bond with one oxygen (o) atom. Possible oxidation states are +2. In writing the electron configuration for magnesium the first two electrons will go. Magnesium Electron Rings.
From montessorimuddle.org
subatomic particles Montessori Muddle Magnesium Electron Rings Possible oxidation states are +2. Magnesium (mg) is able to bond with one oxygen (o) atom. Our magnesium page has over 360 facts that span 117 different quantities. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The final ring or shell of electrons contains the typical number of valence electrons for. Magnesium Electron Rings.
From charlee-yersblogbarnes.blogspot.com
Atomic Mass of Magnesium Magnesium Electron Rings You can see in the dot structure that the two atoms share four different electrons. Magnesium (mg) is able to bond with one oxygen (o) atom. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Our magnesium page has over 360 facts that span 117 different quantities. Magnesium occurs naturally only in.. Magnesium Electron Rings.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Electron Rings Each entry has a full citation identifying its source. Our magnesium page has over 360 facts that span 117 different quantities. Electron configuration of magnesium is [ne] 3s2. Since 1s can only hold two electrons the. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The formula of the. Magnesium Electron Rings.
From diagramdataspongious.z21.web.core.windows.net
Electron Shell Diagram For Carbon Magnesium Electron Rings The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. Electron configuration of magnesium is [ne] 3s2. Magnesium (mg) is able to bond with one oxygen (o) atom. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The electron shells are shown,. Magnesium Electron Rings.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Electron Rings The formula of the compound is mgo. The electron shells are shown, moving outward from the nucleus. Our magnesium page has over 360 facts that span 117 different quantities. Electron configuration of magnesium is [ne] 3s2. Magnesium occurs naturally only in. Since 1s can only hold two electrons the. You can see in the dot structure that the two atoms. Magnesium Electron Rings.