What Is Planning Validation . A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. Validation plans define the scope and goals of a validation project. What is a validation master plan? The validation plan is written at the start of the validation project (sometimes. In addition, it is used to define the timeline for. How to create a validation master plan before the next fda audit?. What is a validation plan. What is a validation master plan (vmp)? A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance.
from www.technolush.com
What is a validation master plan? A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. How to create a validation master plan before the next fda audit?. The validation plan is written at the start of the validation project (sometimes. A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. Validation plans define the scope and goals of a validation project. What is a validation master plan (vmp)? What is a validation plan. In addition, it is used to define the timeline for. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance.
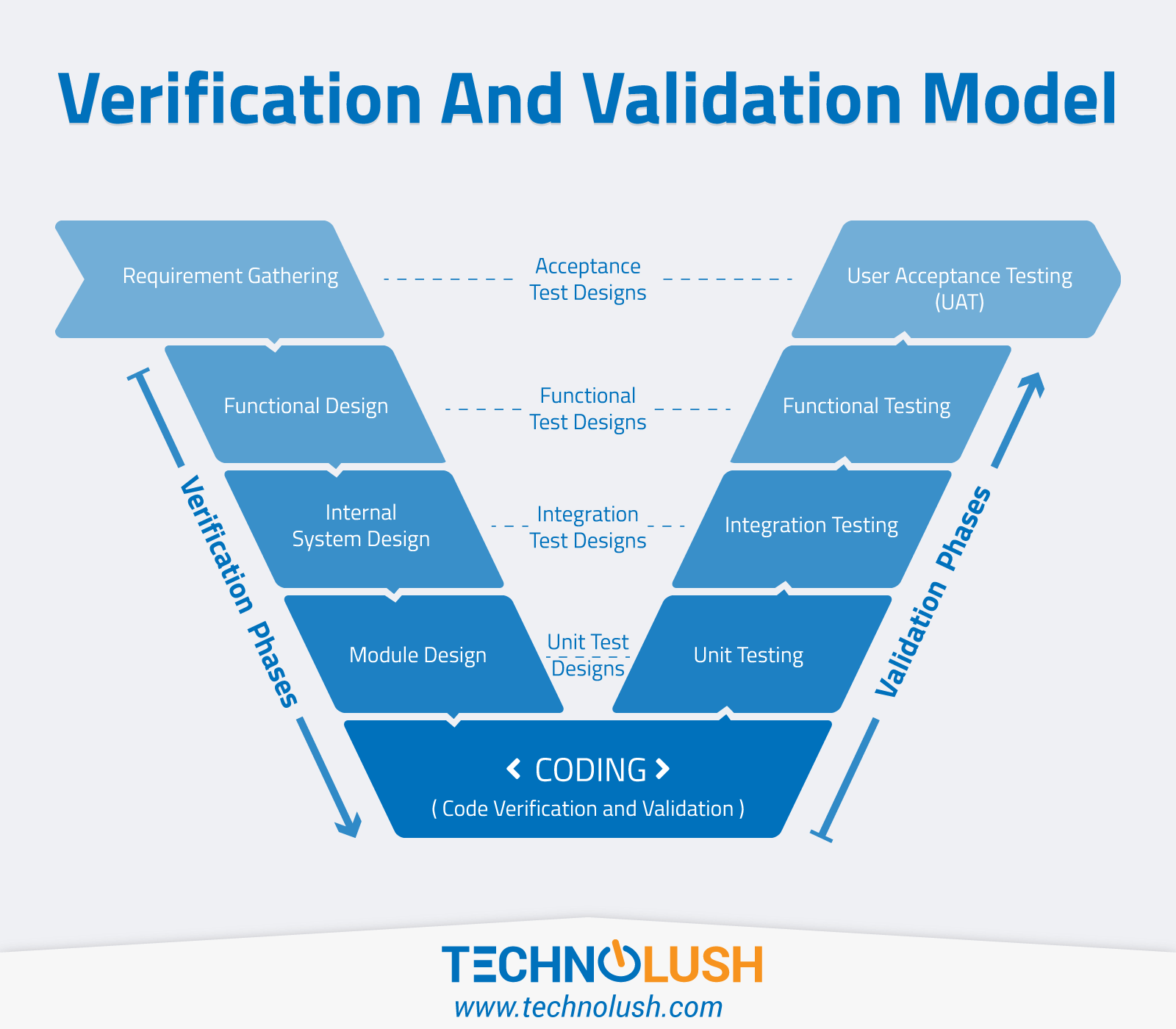
Verification & Validation Model TechnoLush
What Is Planning Validation A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. How to create a validation master plan before the next fda audit?. What is a validation master plan? Validation plans define the scope and goals of a validation project. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. What is a validation master plan (vmp)? The validation plan is written at the start of the validation project (sometimes. In addition, it is used to define the timeline for. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. What is a validation plan.
From www.automationnth.com
Simplifying GAMP Validation Automation NTH Validation Support What Is Planning Validation A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. What is a validation master plan? What is a validation plan. How to create a validation master plan before the next fda audit?. In addition, it is used to define the timeline for. Validation plans define the scope and goals of. What Is Planning Validation.
From www.slideshare.net
Validation plan What Is Planning Validation How to create a validation master plan before the next fda audit?. Validation plans define the scope and goals of a validation project. What is a validation master plan? What is a validation master plan (vmp)? What is a validation plan. The validation plan is written at the start of the validation project (sometimes. A validation master plan, often referred. What Is Planning Validation.
From www.dreamstime.com
Validation Concept Drawn by a Man Stock Illustration Illustration of What Is Planning Validation In addition, it is used to define the timeline for. What is a validation plan. A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. How to create a validation master plan before the next fda audit?. Validation plans define the scope and goals of a validation project. The validation. What Is Planning Validation.
From www.slideteam.net
Design Verification And Validation Process Requirement Management What Is Planning Validation A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. How to create a validation master plan before the next fda audit?. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. What is a validation plan. What is a validation. What Is Planning Validation.
From www.shiksha.com
Difference between Verification and Validation Shiksha Online What Is Planning Validation What is a validation master plan? A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. What is a validation plan. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. The validation plan is written. What Is Planning Validation.
From design.udlvirtual.edu.pe
What Is A Design Validation Plan Design Talk What Is Planning Validation Validation plans define the scope and goals of a validation project. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. A validation master plan, often referred to as vmp, is. What Is Planning Validation.
From www.vrogue.co
What Is Design Qualification Verification And Validat vrogue.co What Is Planning Validation In addition, it is used to define the timeline for. Validation plans define the scope and goals of a validation project. The validation plan is written at the start of the validation project (sometimes. What is a validation master plan (vmp)? What is a validation plan. A vmp is a document that outlines the goals, objectives, processes, and methods for. What Is Planning Validation.
From www.limsforum.com
LIMS Validation Get Yourself Armed with Knowledge This Festive Season What Is Planning Validation What is a validation plan. What is a validation master plan (vmp)? In addition, it is used to define the timeline for. What is a validation master plan? Validation plans define the scope and goals of a validation project. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. How to. What Is Planning Validation.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more What Is Planning Validation In addition, it is used to define the timeline for. What is a validation master plan? The validation plan is written at the start of the validation project (sometimes. What is a validation plan. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. How to create a validation master plan. What Is Planning Validation.
From www.dreamstime.com
Concept of validation stock illustration. Illustration of business What Is Planning Validation Validation plans define the scope and goals of a validation project. In addition, it is used to define the timeline for. What is a validation plan. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. What is a validation master plan? A validation master. What Is Planning Validation.
From www.gmpsop.com
Pharmaceuticals quality assurance & validation procedures GMPSOP What Is Planning Validation What is a validation master plan (vmp)? How to create a validation master plan before the next fda audit?. What is a validation master plan? A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. The validation plan is written at the start of the validation project (sometimes. A validation master. What Is Planning Validation.
From www.slideserve.com
PPT Qualification and Validation PowerPoint Presentation, free What Is Planning Validation A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. What is a validation master plan? The validation plan is written at the start of. What Is Planning Validation.
From www.getreskilled.com
What is a Validation Master Plan (VMP)? GetReskilled What Is Planning Validation A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. Validation plans define the scope and goals of a validation project. The validation plan is written at the start of the validation project (sometimes. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a. What Is Planning Validation.
From www.youtube.com
Validation Master Plan (VMP) YouTube What Is Planning Validation The validation plan is written at the start of the validation project (sometimes. What is a validation master plan (vmp)? What is a validation plan. What is a validation master plan? In addition, it is used to define the timeline for. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of. What Is Planning Validation.
From www.productplan.com
What Is Customer Validation? Definition and Overview ProductPlan What Is Planning Validation In addition, it is used to define the timeline for. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. Validation plans define the scope and goals of a validation project. A vmp is a document that outlines the goals, objectives, processes, and methods for. What Is Planning Validation.
From www.vrogue.co
Validation Master Plan Verification And Validation Qu vrogue.co What Is Planning Validation How to create a validation master plan before the next fda audit?. What is a validation plan. What is a validation master plan (vmp)? What is a validation master plan? The validation plan is written at the start of the validation project (sometimes. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product. What Is Planning Validation.
From www.erp-information.com
What is Validation Master Plan? (Template, Examples) What Is Planning Validation Validation plans define the scope and goals of a validation project. How to create a validation master plan before the next fda audit?. The validation plan is written at the start of the validation project (sometimes. What is a validation master plan (vmp)? A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides. What Is Planning Validation.
From pharmastate.academy
Validation Master Plan for Pharmaceutical Industry What Is Planning Validation A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. What is a validation master plan (vmp)? In addition, it is used to define the timeline for. A validation master plan,. What Is Planning Validation.
From www.presentationeze.com
Overview of the Validation Master Plan PresentationEZE What Is Planning Validation The validation plan is written at the start of the validation project (sometimes. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. In addition, it is used to define the timeline for. Validation plans define the scope and goals of a validation project. A validation master plan (vmp) is a. What Is Planning Validation.
From www.pharmaqualification.com
Validation master plan The Ultimate Guide What Is Planning Validation A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. Validation plans define the scope and goals of a validation project. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. The validation plan is written. What Is Planning Validation.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and What Is Planning Validation A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. What is a validation master plan (vmp)? Validation plans define the scope and goals of a validation project. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment. What Is Planning Validation.
From www.youtube.com
How to Write a Validation Master Plan YouTube What Is Planning Validation What is a validation master plan? A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. In addition, it is used to define the timeline for. What is a validation plan. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of. What Is Planning Validation.
From www.slideshare.net
Validation master plan What Is Planning Validation Validation plans define the scope and goals of a validation project. What is a validation master plan (vmp)? The validation plan is written at the start of the validation project (sometimes. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. How to create a validation master plan before the next. What Is Planning Validation.
From www.erp-information.com
What is Validation Master Plan? (Template, Examples) What Is Planning Validation What is a validation master plan? A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. What is a validation plan. In addition, it is used to define the timeline for. Validation plans define the scope and goals of a validation project. A validation master plan, often referred to as vmp,. What Is Planning Validation.
From www.egnyte.com
Validation Master Plan Egnyte What Is Planning Validation What is a validation master plan? What is a validation master plan (vmp)? The validation plan is written at the start of the validation project (sometimes. What is a validation plan. How to create a validation master plan before the next fda audit?. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides. What Is Planning Validation.
From www.validationtechservices.com
What is a Validation Master Plan An InDepth Guide for Organizational What Is Planning Validation How to create a validation master plan before the next fda audit?. Validation plans define the scope and goals of a validation project. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. What is a validation master plan? The validation plan is written at. What Is Planning Validation.
From radbee.com
Planning for validation Radbee What Is Planning Validation What is a validation master plan (vmp)? A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. What is a validation master plan? What is a validation plan. A validation master. What Is Planning Validation.
From www.gmpsop.com
Nine steps for creating a Master Validation Plan What Is Planning Validation What is a validation plan. In addition, it is used to define the timeline for. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. What is a validation master plan?. What Is Planning Validation.
From www.self-build.co.uk
Planning Permission What is Validation? Build It What Is Planning Validation A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. A vmp is a document that outlines the goals, objectives, processes, and methods for validating. What Is Planning Validation.
From validationcenter.com
What is Computer System Validation and How Do You Do It? What Is Planning Validation What is a validation plan. How to create a validation master plan before the next fda audit?. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. Validation plans define the scope and goals of a validation project. A vmp is a document that outlines. What Is Planning Validation.
From www.erp-information.com
What is Validation Master Plan? (Template, Examples) What Is Planning Validation The validation plan is written at the start of the validation project (sometimes. In addition, it is used to define the timeline for. Validation plans define the scope and goals of a validation project. A validation master plan, often referred to as vmp, is a comprehensive document that defines and provides evidence of an organization’s commitment towards quality assurance. What. What Is Planning Validation.
From www.presentationeze.com
Validation Project Planning PresentationEZE What Is Planning Validation What is a validation plan. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system. Validation plans define the scope and goals of a validation project. In addition, it is used to define the timeline for. The validation plan is written at the start of the validation project (sometimes. What is. What Is Planning Validation.
From operonstrategist.com
Medical Device Validation Master Plan (VMP) Consultancy Firm Operon What Is Planning Validation A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. What is a validation master plan (vmp)? What is a validation master plan? What is a validation plan. How to create a validation master plan before the next fda audit?. Validation plans define the scope and goals of a validation. What Is Planning Validation.
From www.kewaunee.in
HVAC Validation and Qualification for Laboratories Kewaunee What Is Planning Validation In addition, it is used to define the timeline for. Validation plans define the scope and goals of a validation project. A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. How to create a validation master plan before the next fda audit?. A vmp is a document that outlines. What Is Planning Validation.
From www.technolush.com
Verification & Validation Model TechnoLush What Is Planning Validation What is a validation plan. In addition, it is used to define the timeline for. What is a validation master plan? A validation master plan (vmp) is a strategic document that identifies the elements to validate, the approach to each element,. A vmp is a document that outlines the goals, objectives, processes, and methods for validating a product or system.. What Is Planning Validation.