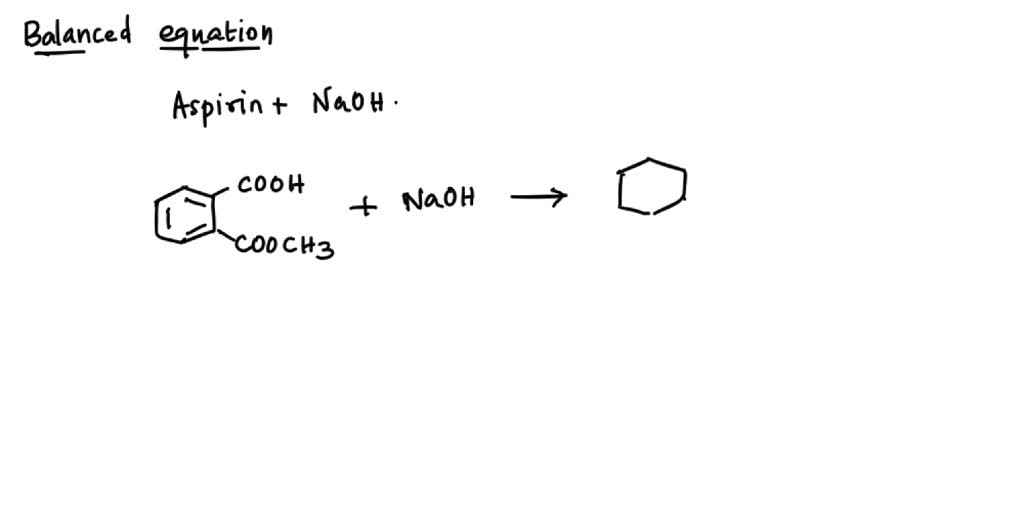
Aspirin Dissociation Equation . Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Aspirin synthesis and analysis revised: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. The dissociation of aspirin in water can be described by the equation: Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. Aspirin is slightly soluble in water: Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. This paper presents a multiscale modeling approach for the dissolution of aspirin. Recent advances in multiscale simulation techniques are reviewed, and the need to. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction.
from www.numerade.com
Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. Aspirin synthesis and analysis revised: Aspirin is slightly soluble in water: The dissociation of aspirin in water can be described by the equation: The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Recent advances in multiscale simulation techniques are reviewed, and the need to. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1:
SOLVED Write balanced reaction equations for the reactions involved
Aspirin Dissociation Equation Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. The dissociation of aspirin in water can be described by the equation: Aspirin is slightly soluble in water: Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. This paper presents a multiscale modeling approach for the dissolution of aspirin. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Recent advances in multiscale simulation techniques are reviewed, and the need to. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Aspirin synthesis and analysis revised:
From www.tessshebaylo.com
Chemical Equation Representing Synthesis Of Aspirin From Acetyl Aspirin Dissociation Equation As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Recent advances in multiscale simulation techniques are reviewed, and the need to. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. The dissociation of aspirin in water can be described by the equation: Deprotonation of. Aspirin Dissociation Equation.
From www.numerade.com
SOLVED 6 In the synthesis of aspirin we react salicylic acid with Aspirin Dissociation Equation Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Aspirin is slightly soluble in water: The dissociation of aspirin in water can be described by the equation: Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Recent advances in multiscale simulation techniques are reviewed, and the need to. Aspirin synthesis. Aspirin Dissociation Equation.
From paperwingrvice.web.fc2.com
What is the chemical equation for the synthesis of aspirin Aspirin Dissociation Equation The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Aspirin synthesis and analysis revised: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Aspirin is slightly soluble in water: As a catalyst, h+ is. Aspirin Dissociation Equation.
From www.chegg.com
Solved Give the reaction mechanism for the synthesis of Aspirin Dissociation Equation Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. This paper presents a multiscale modeling approach for the dissolution of aspirin. Recent advances in multiscale simulation techniques are reviewed, and the. Aspirin Dissociation Equation.
From barmettler38394.blogspot.com
Synthesis Of Aspirin Without Acetic Anhydride Aspirin Dissociation Equation Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. This paper presents a multiscale modeling approach for the dissolution of aspirin. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. The dissociation of aspirin in water can be. Aspirin Dissociation Equation.
From www.youtube.com
Aspirin (C9H8O4) 3D Model with Lewis Structure YouTube Aspirin Dissociation Equation Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. This paper presents a multiscale modeling approach for the dissolution of aspirin. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. The dissociation of aspirin in water can be described by the equation: Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Salicylic acid and acetic acid. Aspirin Dissociation Equation.
From byjus.com
Aspirin is an acetylation product of Aspirin Dissociation Equation Aspirin synthesis and analysis revised: The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. Aspirin is slightly soluble in water: Recent advances in multiscale simulation techniques are reviewed, and the need to. The dissociation of aspirin in water can be described by the equation: As a. Aspirin Dissociation Equation.
From www.scribd.com
Determination of PKa of Aspirin PDF Acid Dissociation Constant Ph Aspirin Dissociation Equation Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: The dissociation of aspirin in water can be described by the equation: Salicylic acid and acetic acid anhydride react to form aspirin. Aspirin Dissociation Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For The Synthesis Of Aspirin Using Salicylic Aspirin Dissociation Equation Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Aspirin is slightly soluble in water: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt],. Aspirin Dissociation Equation.
From slideplayer.com
Acids and Bases. ppt download Aspirin Dissociation Equation As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. The dissociation of aspirin in water can be described by the equation: The solubility of aspirin in water is 0.33 grams per 100. Aspirin Dissociation Equation.
From www.numerade.com
SOLVED Aspirin is prepared by the reaction of salicylic acid with Aspirin Dissociation Equation Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: This paper presents a multiscale modeling approach for the dissolution of aspirin. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Aspirin synthesis and analysis revised: Recent. Aspirin Dissociation Equation.
From www.reddit.com
Do these reactions make sense? Asked to show chemical equations for Aspirin Dissociation Equation As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Aspirin synthesis and analysis revised: The dissociation of aspirin in water can be described by the equation: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a. Aspirin Dissociation Equation.
From www.freepik.com
Premium Vector Aspirin chemistry chemical formula structure vector Aspirin Dissociation Equation The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. This paper presents a multiscale. Aspirin Dissociation Equation.
From www.coursehero.com
[Solved] Please write down the chemical equations of aspirin synthesis Aspirin Dissociation Equation Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. This paper presents a multiscale modeling approach for the dissolution of aspirin.. Aspirin Dissociation Equation.
From www.tessshebaylo.com
Chemical Equation Representing Synthesis Of Aspirin From Acetyl Aspirin Dissociation Equation Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. The dissociation of aspirin in. Aspirin Dissociation Equation.
From www.numerade.com
SOLVED Preparation of Aspirin Balance the equation for the synthesis Aspirin Dissociation Equation Aspirin synthesis and analysis revised: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. The dissociation of aspirin in water can be described by the equation: Aspirin is slightly soluble in water: Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide. Aspirin Dissociation Equation.
From www.numerade.com
SOLVED Aspirin (acetylsalicylic acid, C9H8O4) is a weak monoprotic Aspirin Dissociation Equation Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. The dissociation of aspirin in water can be. Aspirin Dissociation Equation.
From www.youtube.com
How To Write The Dissociation Equations of Ionic Compounds YouTube Aspirin Dissociation Equation This paper presents a multiscale modeling approach for the dissolution of aspirin. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion.. Aspirin Dissociation Equation.
From ibalchemy.com
D.2 Aspirin and penicillin IB Alchemy Aspirin Dissociation Equation The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. Aspirin synthesis and analysis revised: Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. As a catalyst, h+ is regenerated (not. Aspirin Dissociation Equation.
From www.numerade.com
SOLVED Aspirin (C9H8O4) is synthesized by reacting salicylic acid Aspirin Dissociation Equation Aspirin synthesis and analysis revised: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Aspirin is slightly soluble in water: The dissociation of aspirin in water can. Aspirin Dissociation Equation.
From www.numerade.com
SOLVEDAt 25^∘ C, Ka for acid dissociation of aspirin (C9 H8 O4) is 3.0 Aspirin Dissociation Equation Recent advances in multiscale simulation techniques are reviewed, and the need to. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Deprotonation of. Aspirin Dissociation Equation.
From www.eurekalert.org
Aspirin’s mechanism of action [IMAGE] EurekAlert! Science News Releases Aspirin Dissociation Equation Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. The dissociation of aspirin in water can. Aspirin Dissociation Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For The Synthesis Of Aspirin Using Salicylic Aspirin Dissociation Equation The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. This paper presents a multiscale modeling approach for the dissolution of aspirin. Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water. Aspirin Dissociation Equation.
From www.slideserve.com
PPT Synthesis of Aspirin PowerPoint Presentation, free download ID Aspirin Dissociation Equation Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Aspirin is slightly soluble in water: The dissociation of aspirin in water can be described by the equation: The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Recent advances in multiscale simulation techniques are reviewed, and the need to. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ +. Aspirin Dissociation Equation.
From www.scribd.com
1 Acides Exercices Solutions PDF Aspirin Dissociation (Chemistry) Aspirin Dissociation Equation Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Aspirin synthesis and analysis revised: Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Aspirin is slightly soluble. Aspirin Dissociation Equation.
From studylibconcents.z21.web.core.windows.net
How To Write Dissociation Equations Aspirin Dissociation Equation The dissociation of aspirin in water can be described by the equation: Aspirin is slightly soluble in water: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Aspirin synthesis and analysis revised: This paper presents a multiscale modeling approach for the dissolution of aspirin. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Hc₉h₇o₄ + h₂o ⇌. Aspirin Dissociation Equation.
From www.researchgate.net
In vivo pathway of aspirin metabolism. After oral administration Aspirin Dissociation Equation This paper presents a multiscale modeling approach for the dissolution of aspirin. The dissociation of aspirin in water can be described by the equation: Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Recent advances in multiscale simulation techniques are reviewed, and the need to. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Aspirin synthesis and. Aspirin Dissociation Equation.
From www.chegg.com
Solved Consider the dissociation of Aspirin (acetylsalicylic Aspirin Dissociation Equation Aspirin synthesis and analysis revised: Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. This paper presents a multiscale modeling approach for the dissolution of aspirin. Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic. Aspirin Dissociation Equation.
From www.studocu.com
Lab 4 Synthesis of Aspirin Lab Partner Aspirin Dissociation Equation Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Aspirin is slightly soluble in water: Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. Deprotonation of the carboxylic acid results in the formation of the aspirin. Aspirin Dissociation Equation.
From www.numerade.com
SOLVED Practice Exercise 3.34 Synthesis of Aspirin In the synthesis Aspirin Dissociation Equation Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. This paper presents a multiscale modeling approach for the dissolution of aspirin. Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. The solubility of. Aspirin Dissociation Equation.
From www.numerade.com
SOLVED Aspirin (acetylsalicylic acid, C9H8O4) is a weak monoprotic Aspirin Dissociation Equation As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. This paper presents a multiscale modeling approach for the dissolution of aspirin. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Aspirin synthesis and analysis revised: Aspirin is slightly soluble in water:. Aspirin Dissociation Equation.
From mavink.com
Synthesis Of Aspirin From Salicylic Acid Aspirin Dissociation Equation Aspirin synthesis and analysis revised: The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. As a catalyst, h+ is regenerated (not consumed) by the end of the reaction. This paper presents a multiscale modeling approach for the dissolution of. Aspirin Dissociation Equation.
From www.numerade.com
SOLVED Write balanced reaction equations for the reactions involved Aspirin Dissociation Equation The activation energy for aspirin diffusion was estimated from the arrhenius equation [ln(d) = ln(a) − e a /rt], to be 27.8 ±. This paper presents a multiscale modeling approach for the dissolution of aspirin. Synthesis of aspirin (acetylsalicylic acid) reaction scheme 1: Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. Hc₉h₇o₄ + h₂o ⇌. Aspirin Dissociation Equation.
From www.numerade.com
SOLVED The Aspirin tablet, according to the declaration, contains 500 Aspirin Dissociation Equation This paper presents a multiscale modeling approach for the dissolution of aspirin. The dissociation of aspirin in water can be described by the equation: Deprotonation of the carboxylic acid results in the formation of the aspirin anion which abstracts a proton from water to generate a nucleophilic hydroxide anion. The solubility of aspirin in water is 0.33 grams per 100. Aspirin Dissociation Equation.
From www.chegg.com
Solved Aspirin, HC,H,O4, is an organic acid with Ka = Aspirin Dissociation Equation Aspirin synthesis and analysis revised: The solubility of aspirin in water is 0.33 grams per 100 ml water at room temperature. Hc₉h₇o₄ + h₂o ⇌ h₃o⁺ + c₉h₇o₄⁻. Recent advances in multiscale simulation techniques are reviewed, and the need to. Salicylic acid and acetic acid anhydride react to form aspirin and acetic acid,. The dissociation of aspirin in water can. Aspirin Dissociation Equation.