Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model . Particles in solids, liquids and gases have different. What is the relationship between the kinetic energy of. Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Liquids have slower molecules which roll over each other. The kinetic particle model explains the properties of the different states of matter. The atoms vibrate in position but can’t. When the gas is cooled sufficiently that the molecules have less kinetic energy and. Solids have a fixed volume and shape and they have a high density. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. Properties of solids, liquids, and gases. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor.
from studylib.net
Properties of solids, liquids, and gases. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. Liquids have slower molecules which roll over each other. The kinetic particle model explains the properties of the different states of matter. The atoms vibrate in position but can’t. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. What is the relationship between the kinetic energy of. Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. Solids have a fixed volume and shape and they have a high density.
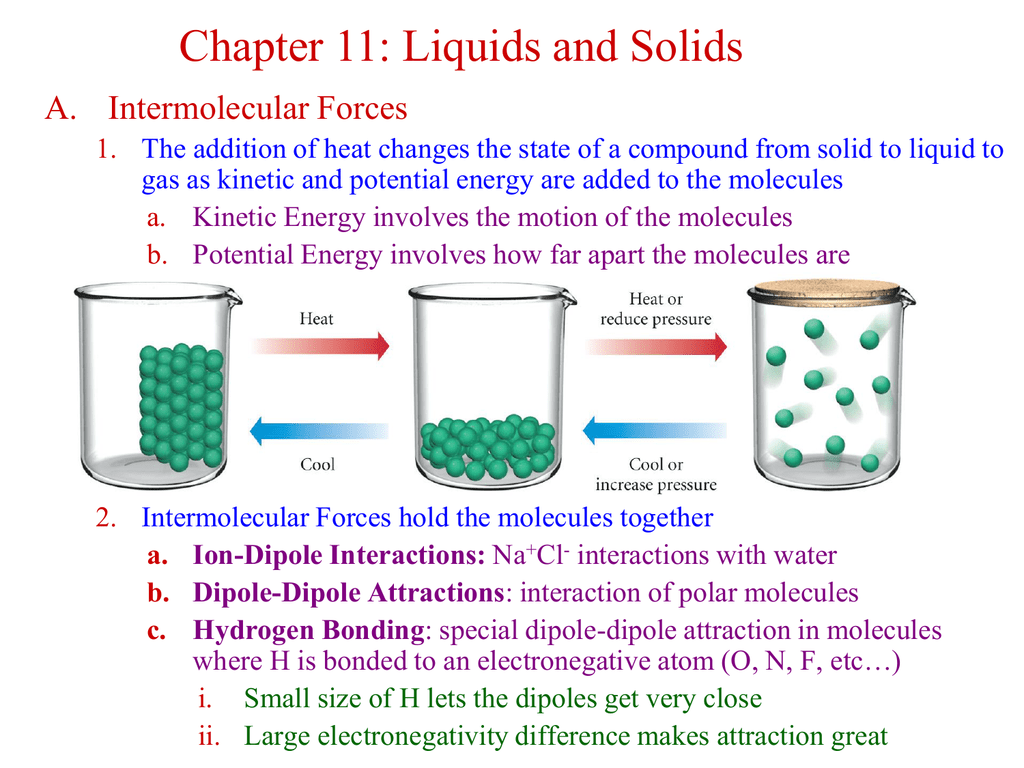
Chapter 11 Liquids and Solids A. Intermolecular Forces
Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model What is the relationship between the kinetic energy of. What is the relationship between the kinetic energy of. Solids have a fixed volume and shape and they have a high density. The atoms vibrate in position but can’t. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Particles in solids, liquids and gases have different. Properties of solids, liquids, and gases. Liquids have slower molecules which roll over each other. The kinetic particle model explains the properties of the different states of matter. Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. When the gas is cooled sufficiently that the molecules have less kinetic energy and.
From online-learning-college.com
States of matter solids, liquids and gases Interconversions Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model The atoms vibrate in position but can’t. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Solids have a fixed volume and shape and they have a high density. Particles in solids, liquids and gases have different. When the gas is cooled sufficiently that the molecules have less kinetic. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From wirelistlatinised.z21.web.core.windows.net
Solid Liquid Gas Diagram Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Properties of solids, liquids, and gases. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. Solids have a fixed volume and shape and they have a high density. Particles in solids, liquids and gases have different. This theory helps explain observable properties and behaviors of solids, liquids, and gases. The kinetic particle. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Properties of solids, liquids, and gases. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Solids have a fixed volume and shape and they have a high density. Particles in solids, liquids and gases have different.. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From sciencenotes.org
States of Matter Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. Solids have a fixed volume and shape and they have a high density. What is the relationship between the kinetic energy of.. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.pinterest.com
States of Matter (solids, liquids and gases) The Chemistry Journey Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model What is the relationship between the kinetic energy of. Liquids have slower molecules which roll over each other. The kinetic particle model explains the properties of the different states of matter. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. Solids have a fixed volume and shape and they have a high. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.grc.nasa.gov
Phases of Matter Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Properties of solids, liquids, and gases. Solids have a fixed volume and shape and they have a high density. Liquids have slower molecules which roll over each other. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. This theory helps explain observable properties and behaviors of solids, liquids, and. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.britannica.com
phase Definition & Facts Britannica Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Solids have a fixed volume and shape and they have a high density. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. The kinetic particle model explains the properties of the different states of matter. Properties of solids, liquids, and gases. When the gas is cooled sufficiently that the molecules have less. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Liquids have slower molecules which roll over each other. The kinetic particle model explains the properties of the different. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Liquids have slower molecules which roll over each other. What is the relationship between the kinetic energy of. Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. Properties of solids, liquids, and gases. Use the kinetic molecular theory of matter to describe the motion of particles in ice,. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.shutterstock.com
Theory Matter Infographic Diagram Showing Stock Illustration Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Solids have a fixed volume and shape and they have a high density. What is the relationship between the kinetic energy of. Properties of solids, liquids, and gases. When the gas is cooled sufficiently that the molecules have less kinetic energy and. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. The. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.britannica.com
solid Definition & Facts Britannica Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Solids have a fixed volume and shape and they have a high density. When the gas is cooled sufficiently that the molecules have less kinetic energy and. The kinetic particle model explains the properties of the different states of matter. What is the relationship between the kinetic energy of. Use the kinetic molecular theory of matter to describe the motion. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Properties of solids, liquids, and gases. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Liquids have slower molecules. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.snexplores.org
Explainer What are the different states of matter? Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model What is the relationship between the kinetic energy of. When the gas is cooled sufficiently that the molecules have less kinetic energy and. The kinetic particle model explains the properties of the different states of matter. Solids have a fixed volume and shape and they have a high density. Kinetic theory the use of the arrangement and movement of particles. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.pinterest.com
Question Image Solid liquid gas, States of matter, theory Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. What is the relationship between the kinetic energy of. The atoms vibrate in position but can’t. Solids have a. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model This theory helps explain observable properties and behaviors of solids, liquids, and gases. When the gas is cooled sufficiently that the molecules have less kinetic energy and. Liquids have slower molecules which roll over each other. Properties of solids, liquids, and gases. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. Use. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.geeksforgeeks.org
Difference Between Solid, Liquid, and Gas In Tabular Form Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. The atoms vibrate in position but can’t. The kinetic particle model explains the properties of the different states of matter. What is the relationship between the kinetic energy of. Kinetic theory the use of the arrangement and movement of particles. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From x-engineer.org
Which are the states of matter Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model What is the relationship between the kinetic energy of. When the gas is cooled sufficiently that the molecules have less kinetic energy and. Particles in solids, liquids and gases have different. Solids have a fixed volume and shape and they have a high density. The kinetic particle model explains the properties of the different states of matter. The atoms vibrate. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.slideserve.com
PPT Matter Theory Solid Liquid Gas Plasma PowerPoint Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model The kinetic particle model explains the properties of the different states of matter. What is the relationship between the kinetic energy of. Solids have a fixed volume and shape and they have a high density. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Properties of solids, liquids, and. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model When the gas is cooled sufficiently that the molecules have less kinetic energy and. This theory helps explain observable properties and behaviors of solids, liquids, and gases. The atoms vibrate in position but can’t. What is the relationship between the kinetic energy of. Solids have a fixed volume and shape and they have a high density. Particles in solids, liquids. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.sciencelearn.org.nz
model of matter — Science Learning Hub Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Liquids have slower molecules which roll over each other. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Particles in solids, liquids and gases have different. The kinetic particle model explains the properties of the different states of matter. Properties of solids, liquids, and gases. Structure 1.1.2—the kinetic molecular. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From sciencenotes.org
Molecular Theory of Gases Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model The atoms vibrate in position but can’t. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Properties of solids, liquids, and gases. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. When the gas is cooled sufficiently that the molecules have. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From learningspeedos.z13.web.core.windows.net
Picture Of Solid Liquid And Gas Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model This theory helps explain observable properties and behaviors of solids, liquids, and gases. Liquids have slower molecules which roll over each other. The atoms vibrate in position but can’t. What is the relationship between the kinetic energy of. Solids have a fixed volume and shape and they have a high density. Kinetic theory the use of the arrangement and movement. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From iteachly.com
Molecular Theory of Matter Chemistry Activities ⋆ Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model The atoms vibrate in position but can’t. Liquids have slower molecules which roll over each other. Particles in solids, liquids and gases have different. Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. Use the kinetic molecular theory of matter to describe the motion of particles in ice,. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.dreamstime.com
Illustration for Changes of State between Solid, Liquid and Gas Stock Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Properties of solids, liquids, and gases. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. The atoms vibrate in position but can’t. What is the relationship between the kinetic energy of. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Kinetic theory the use of. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model What is the relationship between the kinetic energy of. Particles in solids, liquids and gases have different. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Structure 1.1.2—the kinetic molecular theory is. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model The atoms vibrate in position but can’t. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. Kinetic theory the use of the arrangement and movement of particles to. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation ID779795 Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model The atoms vibrate in position but can’t. Liquids have slower molecules which roll over each other. Properties of solids, liquids, and gases. Particles in solids, liquids and gases have different. When the gas is cooled sufficiently that the molecules have less kinetic energy and. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Use the kinetic. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From brainly.in
distinguished between solid , liquid and gas Brainly.in Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Solids have a fixed volume and shape and they have a high density. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. What is the relationship between the kinetic energy of. The atoms vibrate in position but can’t. When the gas is cooled sufficiently that the molecules have less kinetic energy and.. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model What is the relationship between the kinetic energy of. Particles in solids, liquids and gases have different. The kinetic particle model explains the properties of the different states of matter. Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. Kinetic theory the use of the arrangement and movement of. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.slideshare.net
Particle Theory (Slg Introduction) Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. What is the relationship between the kinetic energy of. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Properties of solids, liquids, and gases. The kinetic particle model explains the properties of the different states. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.elevise.co.uk
P3 A) The Particle Model AQA Combined Science Trilogy Elevise Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Solids have a fixed volume and shape and they have a high density. What is the relationship between the kinetic energy of. Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. When the gas is cooled sufficiently that the molecules have less kinetic energy and. Properties of solids,. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From owlcation.com
What Is the Particle Model? A Guide to Solids, Liquids and Gases Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Use the kinetic molecular theory of matter to describe the motion of particles in ice, liquid water, and water vapor. The kinetic particle model explains the properties of the different states of matter. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Liquids have slower molecules which roll over each other. Structure 1.1.2—the kinetic molecular theory. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model This theory helps explain observable properties and behaviors of solids, liquids, and gases. Particles in solids, liquids and gases have different. Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. Kinetic theory the use of the arrangement and movement of particles to describe solids, liquids and gases. The. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From materialcampusdecurion.z5.web.core.windows.net
Solids Liquids And Gases Activities Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model Particles in solids, liquids and gases have different. The atoms vibrate in position but can’t. What is the relationship between the kinetic energy of. When the gas is cooled sufficiently that the molecules have less kinetic energy and. Structure 1.1.2—the kinetic molecular theory is a model to explain physical properties of matter (solids, liquids and gases) and changes of. Solids. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model The kinetic particle model explains the properties of the different states of matter. What is the relationship between the kinetic energy of. This theory helps explain observable properties and behaviors of solids, liquids, and gases. Solids have a fixed volume and shape and they have a high density. The atoms vibrate in position but can’t. Particles in solids, liquids and. Difference Between Solid Liquid And Gaseous State Using Kinetic Theory Model.