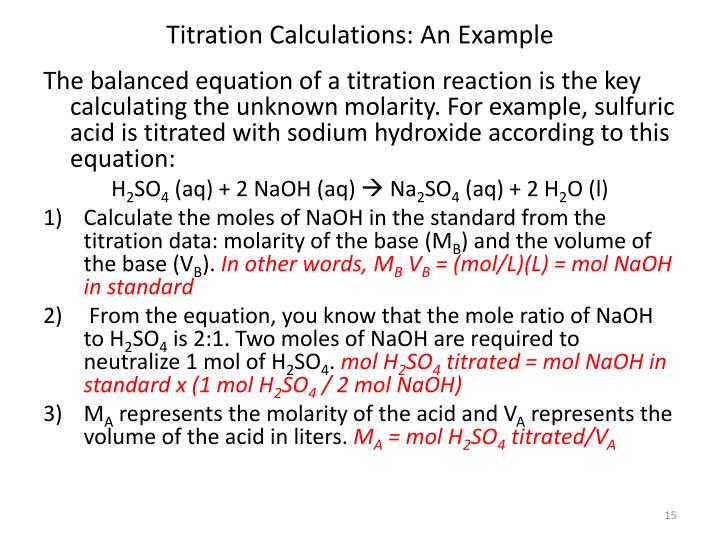
Balanced Equation For Titration Problems . Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Titration between nitric acid and sodium carbonate is represented by the equation. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Calculating the ph during the titration of a weak acid or a weak. Writing a balanced chemical equation. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Complete the calculation in the usual way. The coefficients in the final conversion factor come from the balanced equation for the reaction. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. In the naoh—ch 3 cooh reaction eq. Calculating the ph of a solution of a weak acid or a weak base.
from www.slideserve.com
The coefficients in the final conversion factor come from the balanced equation for the reaction. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration between nitric acid and sodium carbonate is represented by the equation. Writing a balanced chemical equation. In the naoh—ch 3 cooh reaction eq. Calculating the ph during the titration of a weak acid or a weak. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Complete the calculation in the usual way.
PPT Titrations PowerPoint Presentation ID5572517
Balanced Equation For Titration Problems The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In the naoh—ch 3 cooh reaction eq. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: The coefficients in the final conversion factor come from the balanced equation for the reaction. Complete the calculation in the usual way. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Titration between nitric acid and sodium carbonate is represented by the equation. Calculating the ph during the titration of a weak acid or a weak. Calculating the ph of a solution of a weak acid or a weak base. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Writing a balanced chemical equation.
From www.chegg.com
Balanced equation for the titration reaction in the Balanced Equation For Titration Problems Writing a balanced chemical equation. The coefficients in the final conversion factor come from the balanced equation for the reaction. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Calculating the ph of a solution of a weak acid or a weak base. The object of a titration is always to add just the amount of titrant needed to. Balanced Equation For Titration Problems.
From www.numerade.com
SOLVED Consider different titrations for this exercise. Potassium Balanced Equation For Titration Problems Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Calculating the ph during the titration of a weak acid or a weak. Writing a balanced chemical equation. Calculate the ph. Balanced Equation For Titration Problems.
From www.vrogue.co
Solved The Balanced Equation For The Titration Of Oxa vrogue.co Balanced Equation For Titration Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Titration between nitric acid and sodium carbonate is represented by the equation. Complete the calculation in the usual way. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Naoh. Balanced Equation For Titration Problems.
From www.slideserve.com
PPT Steps for solving titration problems PowerPoint Presentation Balanced Equation For Titration Problems The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base:. Balanced Equation For Titration Problems.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Balanced Equation For Titration Problems Calculating the ph during the titration of a weak acid or a weak. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. The coefficients in the final conversion factor come from the balanced equation for the reaction. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Complete the calculation in the usual way.. Balanced Equation For Titration Problems.
From www.youtube.com
Acid Base Neutralization Reactions & Net Ionic Equations Chemistry Balanced Equation For Titration Problems The coefficients in the final conversion factor come from the balanced equation for the reaction. Complete the calculation in the usual way. In the naoh—ch 3 cooh reaction eq. Calculating the ph during the titration of a weak acid or a weak. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. 2h n o3(aq)+. Balanced Equation For Titration Problems.
From www.slideserve.com
PPT Titrations PowerPoint Presentation ID5572517 Balanced Equation For Titration Problems The coefficients in the final conversion factor come from the balanced equation for the reaction. In the naoh—ch 3 cooh reaction eq. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculating the ph of a solution of a weak acid or a weak base. 2h n o3(aq)+ n. Balanced Equation For Titration Problems.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Balanced Equation For Titration Problems Calculating the ph of a solution of a weak acid or a weak base. Titration between nitric acid and sodium carbonate is represented by the equation. In the naoh—ch 3 cooh reaction eq. Complete the calculation in the usual way. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Calculate the ph for the strong acid/strong base titration between. Balanced Equation For Titration Problems.
From www.chegg.com
Solved To review how titration calculations work, here's a Balanced Equation For Titration Problems Complete the calculation in the usual way. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculating the ph of a solution of a weak acid or a weak base. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and. Balanced Equation For Titration Problems.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube Balanced Equation For Titration Problems Calculating the ph during the titration of a weak acid or a weak. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculating the ph of a solution of a weak acid or a weak base. Calculate the ph for the strong acid/strong base titration between 50.0 ml of. Balanced Equation For Titration Problems.
From www.youtube.com
acids and bases examples of titration problems YouTube Balanced Equation For Titration Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Titration between nitric acid and sodium carbonate is represented by the equation. Complete the calculation in the usual way. Naoh (aq) + hcl (aq) → nacl (aq) + h 2. Balanced Equation For Titration Problems.
From www.numerade.com
SOLVED A sample of ascorbic acid, with the formula C6H8O6, is to be Balanced Equation For Titration Problems Titration between nitric acid and sodium carbonate is represented by the equation. Calculating the ph of a solution of a weak acid or a weak base. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: The above equation works. Balanced Equation For Titration Problems.
From www.numerade.com
SOLVED 1. Gianna performed acidbase titration to determine the Balanced Equation For Titration Problems Writing a balanced chemical equation. The coefficients in the final conversion factor come from the balanced equation for the reaction. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In the naoh—ch 3 cooh reaction eq. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l). Balanced Equation For Titration Problems.
From www.numerade.com
SOLVED Write a balanced equation for the corresponding titration Balanced Equation For Titration Problems Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: In the naoh—ch 3 cooh reaction eq. Titration between nitric acid and sodium carbonate is. Balanced Equation For Titration Problems.
From www.chegg.com
Solved Consider a different titration for this exercise. Balanced Equation For Titration Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +.. Balanced Equation For Titration Problems.
From shotprofessional22.gitlab.io
Ace Khp And Naoh Balanced Equation Physics Wallah 12th Class Chemistry Pdf Balanced Equation For Titration Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Complete the calculation in the usual way. Titration between nitric acid and sodium carbonate is represented by the equation. Naoh (aq) + hcl (aq) → nacl (aq) + h 2. Balanced Equation For Titration Problems.
From www.tessshebaylo.com
Find Empirical Formula From Combustion Equation Tessshebaylo Balanced Equation For Titration Problems Complete the calculation in the usual way. In the naoh—ch 3 cooh reaction eq. Calculating the ph of a solution of a weak acid or a weak base. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Writing a balanced chemical equation. The coefficients in the final conversion factor. Balanced Equation For Titration Problems.
From www.youtube.com
Titration Calculations YouTube Balanced Equation For Titration Problems Calculating the ph during the titration of a weak acid or a weak. Complete the calculation in the usual way. Titration between nitric acid and sodium carbonate is represented by the equation. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of. Balanced Equation For Titration Problems.
From mavink.com
Acid Base Titration Equation Balanced Equation For Titration Problems Titration between nitric acid and sodium carbonate is represented by the equation. Writing a balanced chemical equation. In the naoh—ch 3 cooh reaction eq. Calculating the ph of a solution of a weak acid or a weak base. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Complete the. Balanced Equation For Titration Problems.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Balanced Equation For Titration Problems The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. The coefficients in the final conversion factor come from the balanced equation for the reaction. Calculating the ph of a solution of a weak acid or a weak base. Complete the calculation in the usual way.. Balanced Equation For Titration Problems.
From ar.inspiredpencil.com
Titration Equation Balanced Equation For Titration Problems Complete the calculation in the usual way. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Calculating the ph of a solution of a weak acid or a weak base. Naoh (aq) + hcl (aq) → nacl (aq) +. Balanced Equation For Titration Problems.
From www.numerade.com
SOLVED More Titration Problems Consider the titration of 25.00 mL of Balanced Equation For Titration Problems In the naoh—ch 3 cooh reaction eq. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Calculating the ph of a solution of a weak acid or a weak base. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the.. Balanced Equation For Titration Problems.
From www.nagwa.com
Question Video Calculating the Mass of Solute in a Solution via Balanced Equation For Titration Problems Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Calculating the ph of a solution of a weak acid or a weak base. The coefficients in the final conversion factor come from the balanced equation for the reaction. Complete the calculation in the usual way. The above equation works only for neutralizations in which. Balanced Equation For Titration Problems.
From www.numerade.com
SOLVED Given this balanced chemical equation and the following Balanced Equation For Titration Problems Calculating the ph of a solution of a weak acid or a weak base. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculating the. Balanced Equation For Titration Problems.
From www.numerade.com
SOLVED Write the equation for the titration of Ca2+ with EDTA. Balanced Equation For Titration Problems The coefficients in the final conversion factor come from the balanced equation for the reaction. Complete the calculation in the usual way. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculate the ph for the strong acid/strong base. Balanced Equation For Titration Problems.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Balanced Equation For Titration Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Titration between nitric acid and sodium carbonate is represented by the equation. The coefficients in the final conversion factor come from the balanced equation for the reaction. The object of. Balanced Equation For Titration Problems.
From www.vrogue.co
Solved The Balanced Equation For The Titration Of Oxa vrogue.co Balanced Equation For Titration Problems In the naoh—ch 3 cooh reaction eq. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Calculating the ph during the titration of a weak acid or a weak.. Balanced Equation For Titration Problems.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Balanced Equation For Titration Problems In the naoh—ch 3 cooh reaction eq. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Complete the calculation in the usual way. Calculating the ph of a solution of a weak acid or a weak base. Titration between nitric acid and sodium carbonate. Balanced Equation For Titration Problems.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Balanced Equation For Titration Problems Calculating the ph during the titration of a weak acid or a weak. Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. In the naoh—ch 3 cooh reaction eq. Titration between nitric acid and sodium carbonate is represented by the equation. The coefficients in the final conversion factor come from the balanced equation for. Balanced Equation For Titration Problems.
From www.studypool.com
SOLUTION General Chemistry Sample Problems (Concentration to Titration Balanced Equation For Titration Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: Naoh (aq) + hcl (aq) → nacl (aq) + h 2 o (l) so the. Complete the calculation in the usual way. Calculating the ph of a solution of a. Balanced Equation For Titration Problems.
From unacademy.com
Mohr salt titration Balanced Equation For Titration Problems In the naoh—ch 3 cooh reaction eq. Titration between nitric acid and sodium carbonate is represented by the equation. Calculating the ph during the titration of a weak acid or a weak. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of. Balanced Equation For Titration Problems.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with Balanced Equation For Titration Problems In the naoh—ch 3 cooh reaction eq. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed volumes of added base: 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Writing a balanced chemical equation. The object of a titration is always to. Balanced Equation For Titration Problems.
From mungfali.com
Acid Base Titration Calculation Balanced Equation For Titration Problems 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. Calculating the ph during the titration of a weak acid or a weak. The coefficients in the final conversion factor come from the balanced equation for. Balanced Equation For Titration Problems.
From studylib.net
AcidBase Titration Answers, Sources of Error Balanced Equation For Titration Problems The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Calculating the ph during the titration of a weak acid or a weak. Complete the calculation in the usual way. Titration between nitric acid and sodium carbonate is represented by. Balanced Equation For Titration Problems.
From www.vrogue.co
Acid Base Titration Problems Basic Introduction Calcu vrogue.co Balanced Equation For Titration Problems Calculating the ph during the titration of a weak acid or a weak. The object of a titration is always to add just the amount of titrant needed to consume exactly the amount of substance being titrated. 2h n o3(aq)+ n a2co3(aq) → n a(n o3)2(aq) +. Titration between nitric acid and sodium carbonate is represented by the equation. Naoh. Balanced Equation For Titration Problems.