What Is The Most Ionic Element . When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When the atom loses electron/s, it forms positively charged ion. Looking for a periodic table of elements with common charges? Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. This electric charge generated on the ion is. 93 rows ionic charge: Ions are formed as a result of this addition or removal of electrons from the atom. Unlike protons and neutrons, electrons can be easily removed and added to an atom. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. This table contains the most common.
from www.youtube.com
Ions are formed as a result of this addition or removal of electrons from the atom. 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When the atom loses electron/s, it forms positively charged ion. Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Looking for a periodic table of elements with common charges? This table contains the most common. This electric charge generated on the ion is. Unlike protons and neutrons, electrons can be easily removed and added to an atom.
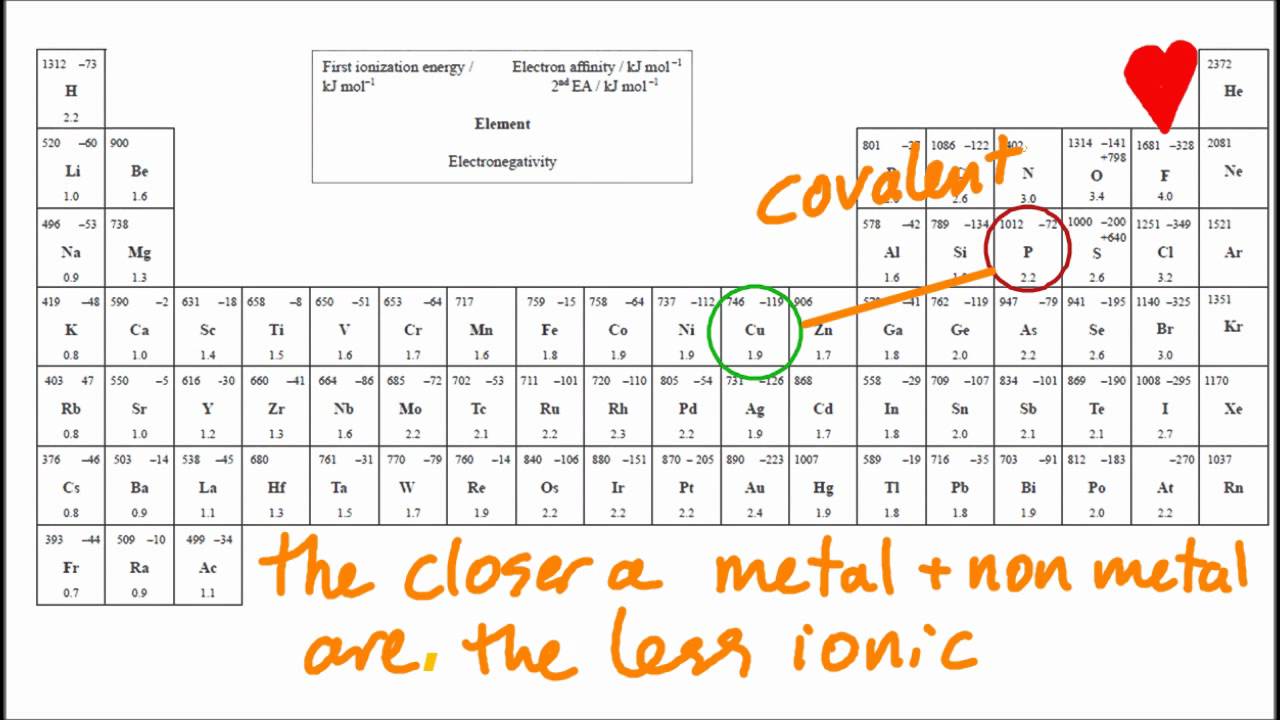
4.2 Predict if a compound of 2 elements is ionic using the table of EN
What Is The Most Ionic Element Ions are formed as a result of this addition or removal of electrons from the atom. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. Ions are formed as a result of this addition or removal of electrons from the atom. 93 rows ionic charge: Unlike protons and neutrons, electrons can be easily removed and added to an atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This table contains the most common. Looking for a periodic table of elements with common charges? Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. When the atom loses electron/s, it forms positively charged ion. This electric charge generated on the ion is.
From science4geeks.blogspot.com
Science4Geeks Element, Compound and Mixture What Is The Most Ionic Element This electric charge generated on the ion is. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. Unlike protons and neutrons, electrons can be easily removed and added to an atom. Looking for a periodic table of elements with common charges? Because the number of protons remains unchanged when an atom. What Is The Most Ionic Element.
From sciencenotes.org
Atomic Radius and Ionic Radius What Is The Most Ionic Element When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Ions are formed as a result of this addition or removal of electrons from the atom. Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. This electric charge generated on. What Is The Most Ionic Element.
From utedzz.blogspot.com
Ionic Radius Values Periodic Table Periodic Table Timeline What Is The Most Ionic Element Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. This electric charge generated on the ion is. Unlike protons and neutrons, electrons can be easily removed and added to an atom. Ionic charge is the electrical charge that is generated on the atom when it loses or. What Is The Most Ionic Element.
From socratic.org
Which Chloride should have the greatest covalent character? Socratic What Is The Most Ionic Element Looking for a periodic table of elements with common charges? Unlike protons and neutrons, electrons can be easily removed and added to an atom. When the atom loses electron/s, it forms positively charged ion. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Ionic charge is the electrical charge. What Is The Most Ionic Element.
From chem.libretexts.org
3.1 Ionic Atoms Chemistry LibreTexts What Is The Most Ionic Element This electric charge generated on the ion is. Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. When the atom loses or gains one or more electrons, the electric charge is generated. What Is The Most Ionic Element.
From chemistryismyjam.com
Chemical Bonding Chemistry Is My Jam! What Is The Most Ionic Element This electric charge generated on the ion is. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Looking for a periodic table of elements with common charges? Unlike protons and neutrons, electrons can be easily removed and added to an atom. Ionic charge is the electrical charge. What Is The Most Ionic Element.
From www.youtube.com
How To Determine The Charge of Elements and Ions Chemistry YouTube What Is The Most Ionic Element Ions are formed as a result of this addition or removal of electrons from the atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. Ionic charge is the electrical charge that is generated on the atom when it loses or. What Is The Most Ionic Element.
From utedzz.blogspot.com
Printable Periodic Table Ionic Charges Periodic Table Timeline What Is The Most Ionic Element This electric charge generated on the ion is. Ions are formed as a result of this addition or removal of electrons from the atom. Unlike protons and neutrons, electrons can be easily removed and added to an atom. 93 rows ionic charge: Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the. What Is The Most Ionic Element.
From socratic.org
How can you tell if an element wants to gain or lose electrons? Socratic What Is The Most Ionic Element Unlike protons and neutrons, electrons can be easily removed and added to an atom. Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. This electric charge generated on the ion is. When the atom loses electron/s, it forms positively charged ion. Ions are formed as a result of this addition. What Is The Most Ionic Element.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry What Is The Most Ionic Element Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Unlike protons and neutrons, electrons can be easily removed and added to an atom. Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. Ionic charge is the electrical. What Is The Most Ionic Element.
From www.nemoquiz.com
Ion Charge from Periodic Table NemoQuiz What Is The Most Ionic Element Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. Looking for a periodic table of elements with common charges? This electric charge generated on the ion is. 93 rows ionic charge: Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element. What Is The Most Ionic Element.
From www.showme.com
ShowMe elements in the periodic table What Is The Most Ionic Element Ions are formed as a result of this addition or removal of electrons from the atom. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. Looking for a periodic table of elements with common charges? This table contains the most common. This electric charge generated on the ion is. 93 rows. What Is The Most Ionic Element.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination What Is The Most Ionic Element 93 rows ionic charge: Ions are formed as a result of this addition or removal of electrons from the atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When the atom loses electron/s, it forms positively charged ion. Ionic charge is the electrical charge that is generated on. What Is The Most Ionic Element.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend What Is The Most Ionic Element When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s.. What Is The Most Ionic Element.
From sciencenotes.org
Periodic Table of Element Atom Sizes What Is The Most Ionic Element Unlike protons and neutrons, electrons can be easily removed and added to an atom. This table contains the most common. Looking for a periodic table of elements with common charges? Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. Based on the common charges, we know iron can lose two or. What Is The Most Ionic Element.
From learnwithdrscott.com
Ionic vs Covalent Easy Hard Science What Is The Most Ionic Element Ions are formed as a result of this addition or removal of electrons from the atom. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. This electric charge generated. What Is The Most Ionic Element.
From courses.lumenlearning.com
Molecular and Ionic Compounds Chemistry I What Is The Most Ionic Element Unlike protons and neutrons, electrons can be easily removed and added to an atom. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. Looking for a periodic table of elements with common. What Is The Most Ionic Element.
From www.youtube.com
Naming Simple Ionic Compounds YouTube What Is The Most Ionic Element This table contains the most common. Looking for a periodic table of elements with common charges? Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Unlike protons and neutrons, electrons can be easily removed and added to an atom. When the atom loses or gains one or. What Is The Most Ionic Element.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii What Is The Most Ionic Element Looking for a periodic table of elements with common charges? 93 rows ionic charge: When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When the atom loses electron/s, it forms positively charged ion. Unlike protons and neutrons, electrons can be easily removed and added to an atom. Ionic charge. What Is The Most Ionic Element.
From sites.google.com
Periodic Table of Elements & Ionic Bonding Mrs. Zeringue's 7th Grade What Is The Most Ionic Element When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be. What Is The Most Ionic Element.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry What Is The Most Ionic Element Looking for a periodic table of elements with common charges? Ions are formed as a result of this addition or removal of electrons from the atom. 93 rows ionic charge: Unlike protons and neutrons, electrons can be easily removed and added to an atom. When the atom loses electron/s, it forms positively charged ion. Based on the common charges, we. What Is The Most Ionic Element.
From www.youtube.com
4.2 Predict if a compound of 2 elements is ionic using the table of EN What Is The Most Ionic Element When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. Ions are formed as a result of this addition or removal of electrons from the atom. This table contains the most common. Based on the common charges, we know iron can lose. What Is The Most Ionic Element.
From www.pathwaystochemistry.com
Naming Simple Ionic Compounds Pathways to Chemistry What Is The Most Ionic Element This table contains the most common. Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Unlike protons and neutrons, electrons can be easily removed and added to an atom. Because. What Is The Most Ionic Element.
From www.flinnsci.com
Ion Names, Formulas, and Charges Charts for Chemistry What Is The Most Ionic Element Unlike protons and neutrons, electrons can be easily removed and added to an atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Based on the common charges, we know iron can lose two or three electrons to achieve a stable electron. This table contains the most common. Ionic. What Is The Most Ionic Element.
From utedzz.blogspot.com
Periodic Table Ion Charges Periodic Table Timeline What Is The Most Ionic Element When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Ions are formed as a result of this addition or removal of electrons from the atom. 93 rows ionic charge: This table contains the most common. Unlike protons and neutrons, electrons can be easily removed and added to an atom.. What Is The Most Ionic Element.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica What Is The Most Ionic Element This table contains the most common. Looking for a periodic table of elements with common charges? This electric charge generated on the ion is. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Based on the common charges, we know iron can lose two or three electrons. What Is The Most Ionic Element.
From education-portal.com
What Are Ionic Compounds? Definition, Examples & Reactions Video What Is The Most Ionic Element Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Unlike protons and neutrons, electrons can be easily removed and added to an atom. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When the atom loses. What Is The Most Ionic Element.
From superhumanacademy.com
How to Memorize Polyatomic Ions & Chemical Formulas SuperHuman Academy What Is The Most Ionic Element Looking for a periodic table of elements with common charges? This table contains the most common. Unlike protons and neutrons, electrons can be easily removed and added to an atom. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. 93 rows ionic charge: Because the number of protons remains unchanged when. What Is The Most Ionic Element.
From courses.lumenlearning.com
Elements and Atoms The Building Blocks of Matter Anatomy and What Is The Most Ionic Element When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). Looking for a periodic table of elements with common charges? Ions are formed as a result of this addition or removal of electrons from the atom. Based on the common charges, we know iron can lose two or three electrons. What Is The Most Ionic Element.
From www.compoundchem.com
Compound Interest 10 Periodic Table of Common Ions What Is The Most Ionic Element When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). When the atom loses electron/s, it forms positively charged ion. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. Unlike protons and neutrons, electrons can be easily removed and added to. What Is The Most Ionic Element.
From zakruti.com
Example naming ionic compound What Is The Most Ionic Element When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This electric charge generated on the ion is. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. This table contains the most common. Based on the common. What Is The Most Ionic Element.
From www.masterorganicchemistry.com
Ionic and Covalent Bonding Master Organic Chemistry What Is The Most Ionic Element This electric charge generated on the ion is. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). This table contains the most common. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. Because the number of protons remains unchanged when. What Is The Most Ionic Element.
From www.animalia-life.club
Ionic Compounds Periodic Table What Is The Most Ionic Element Unlike protons and neutrons, electrons can be easily removed and added to an atom. 93 rows ionic charge: Ions are formed as a result of this addition or removal of electrons from the atom. When the atom loses electron/s, it forms positively charged ion. Looking for a periodic table of elements with common charges? Based on the common charges, we. What Is The Most Ionic Element.
From elchoroukhost.net
Periodic Table With Charges Of Ions Elcho Table What Is The Most Ionic Element Ions are formed as a result of this addition or removal of electrons from the atom. Ionic charge is the electrical charge that is generated on the atom when it loses or gains electron/s. Because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Unlike protons and neutrons,. What Is The Most Ionic Element.
From www.animalia-life.club
Ionic Compounds Periodic Table What Is The Most Ionic Element When the atom loses electron/s, it forms positively charged ion. When the atom loses or gains one or more electrons, the electric charge is generated (and an ion is formed). 93 rows ionic charge: Looking for a periodic table of elements with common charges? Based on the common charges, we know iron can lose two or three electrons to achieve. What Is The Most Ionic Element.