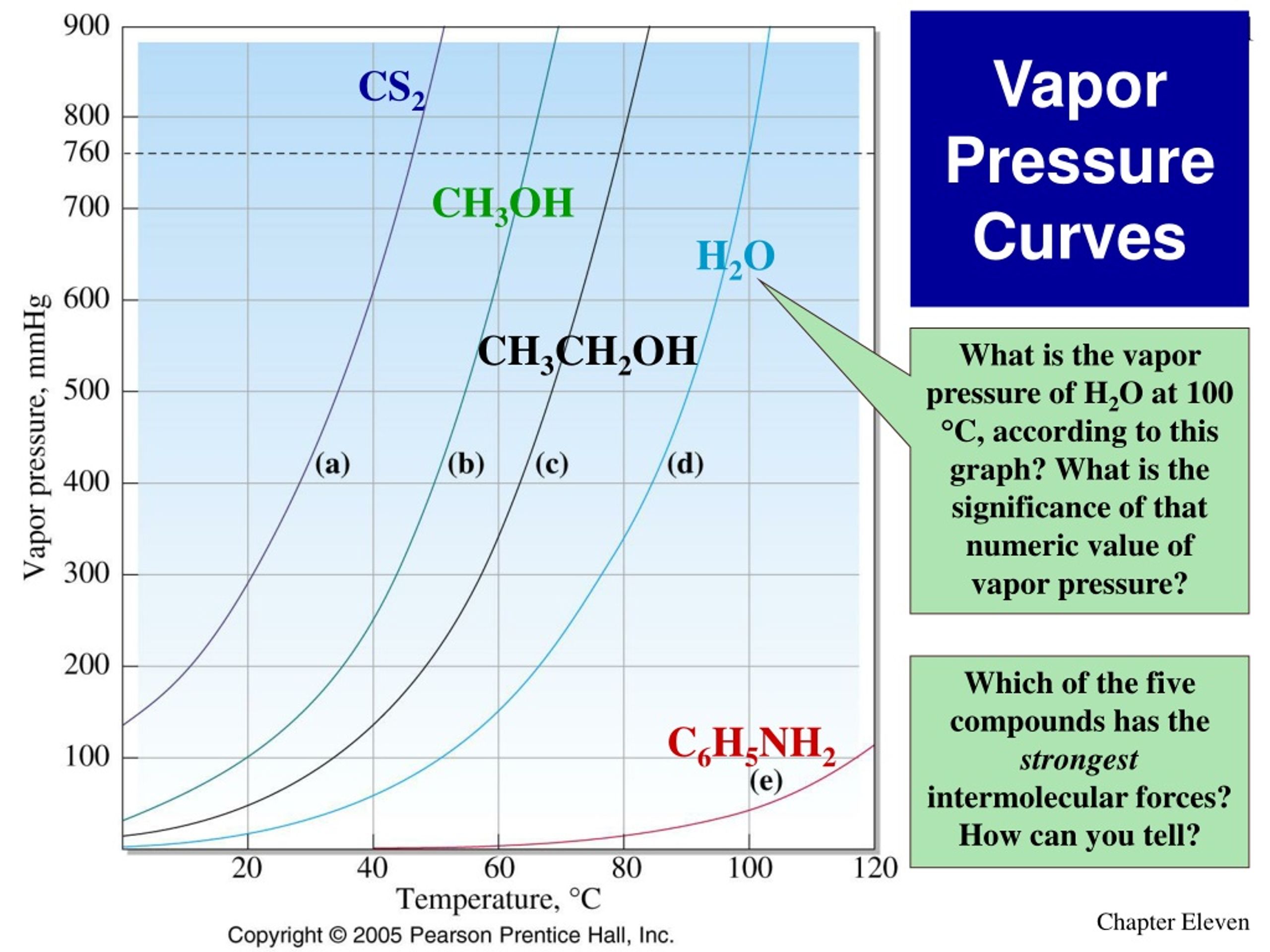
Y Does Vapor Pressure Increase With Temperature . the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. the increase in vapor pressure with temperature is not linear, however. The line on the graph shows the boiling. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in.
from www.slideserve.com
the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. The line on the graph shows the boiling. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. the increase in vapor pressure with temperature is not linear, however. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.
PPT States of Matter and Intermolecular Forces PowerPoint
Y Does Vapor Pressure Increase With Temperature in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. The line on the graph shows the boiling. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the increase in vapor pressure with temperature is not linear, however. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water.
From dxopxntvm.blob.core.windows.net
Why Does Water Vapor Pressure Increase With Temperature at Antonio Y Does Vapor Pressure Increase With Temperature in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Y Does Vapor Pressure Increase With Temperature.
From classnotes.org.in
Vapour Pressure Chemistry, Class 11, States of Matter Y Does Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. The line on the graph shows the boiling. . Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Y Does Vapor Pressure Increase With Temperature the increase in vapor pressure with temperature is not linear, however. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. For example, consider the vapor pressure. Y Does Vapor Pressure Increase With Temperature.
From saylordotorg.github.io
Vapor Pressure Y Does Vapor Pressure Increase With Temperature as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. The line on the graph shows the boiling. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the increase in vapor pressure with temperature is not. Y Does Vapor Pressure Increase With Temperature.
From socratic.org
What is the relation between critical temperature and boiling point or Y Does Vapor Pressure Increase With Temperature in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Y Does Vapor Pressure Increase With Temperature.
From www.chemistrylibrary.org
Vapor Pressure Y Does Vapor Pressure Increase With Temperature the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. as the temperature of a liquid increases, the vapor pressure. Y Does Vapor Pressure Increase With Temperature.
From schoolbag.info
5 a read the graph and add the two pressures Y Does Vapor Pressure Increase With Temperature the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. The line on the graph shows the boiling. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e.. Y Does Vapor Pressure Increase With Temperature.
From shaunmwilliams.com
Chapter 10 Presentation Y Does Vapor Pressure Increase With Temperature The line on the graph shows the boiling. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the increase in vapor pressure with temperature is not linear, however. For example, consider the vapor pressure. Y Does Vapor Pressure Increase With Temperature.
From www.youtube.com
Vapor pressure versus temperature relations of common elements YouTube Y Does Vapor Pressure Increase With Temperature the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. the increase in vapor pressure with temperature is not linear, however. as the temperature of a liquid increases, the vapor pressure of the liquid increases. Y Does Vapor Pressure Increase With Temperature.
From saylordotorg.github.io
Vapor Pressure Y Does Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. the increase in vapor pressure with temperature is not linear, however. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. The line on the graph shows the. Y Does Vapor Pressure Increase With Temperature.
From socratic.org
Explain how each of the following affects the vapour pressure of a Y Does Vapor Pressure Increase With Temperature The line on the graph shows the boiling. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. the increase in vapor pressure with temperature is not linear, however. For example, consider the vapor pressure of water between 0°c and 100 °c, shown. Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint Y Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. the increase in vapor pressure with temperature is not linear, however. The line on the graph shows the boiling. as the temperature of a liquid increases, the. Y Does Vapor Pressure Increase With Temperature.
From www.researchgate.net
Vapor pressure and density of water vapor as function of temperature Y Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. The line on the graph shows the boiling. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. as the. Y Does Vapor Pressure Increase With Temperature.
From slidetodoc.com
Aim How does temperature affect the vapor pressure Y Does Vapor Pressure Increase With Temperature in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the increase in vapor pressure with temperature is not linear, however. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. as the temperature of a liquid increases, the vapor pressure of the liquid. Y Does Vapor Pressure Increase With Temperature.
From www.numerade.com
SOLVED This graph shows how three liquids' vapor pressure changes with Y Does Vapor Pressure Increase With Temperature the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. as the temperature of a liquid increases, the vapor pressure. Y Does Vapor Pressure Increase With Temperature.
From dxopxntvm.blob.core.windows.net
Why Does Water Vapor Pressure Increase With Temperature at Antonio Y Does Vapor Pressure Increase With Temperature as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. in an open container, vapor pressure rises as temperature increases until the temperature reaches the. Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Y Does Vapor Pressure Increase With Temperature in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. The line on the graph shows the boiling. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. . Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Y Does Vapor Pressure Increase With Temperature in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. . Y Does Vapor Pressure Increase With Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Y Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the increase in vapor pressure with temperature is not linear,. Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID1025977 Y Does Vapor Pressure Increase With Temperature For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. the increase in vapor pressure with temperature is not linear, however. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling. Y Does Vapor Pressure Increase With Temperature.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Y Does Vapor Pressure Increase With Temperature in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Y Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The line on the graph shows the boiling. the increase in vapor pressure with temperature is not linear, however. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. in an open container, vapor. Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Y Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water.. Y Does Vapor Pressure Increase With Temperature.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Y Does Vapor Pressure Increase With Temperature the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. generally a substance's vapor pressure increases as temperature. Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Ch. 11 Liquids, Solids, and Intermolecular Forces PowerPoint Y Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The line on the graph shows the boiling. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water.. Y Does Vapor Pressure Increase With Temperature.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids Y Does Vapor Pressure Increase With Temperature the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. the increase in vapor pressure with temperature is not linear, however. The line on the graph shows the boiling. For example, consider the vapor pressure of water between 0°c and 100 °c, shown in. in an open container, vapor pressure. Y Does Vapor Pressure Increase With Temperature.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel Y Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. the increase in vapor pressure with temperature is not linear, however. as the temperature of a liquid increases, the vapor pressure of the liquid. Y Does Vapor Pressure Increase With Temperature.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor Y Does Vapor Pressure Increase With Temperature The line on the graph shows the boiling. the increase in vapor pressure with temperature is not linear, however. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. generally a substance's. Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID2755893 Y Does Vapor Pressure Increase With Temperature as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. The line on the graph shows the boiling. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the vapor pressure of a liquid varies with its. Y Does Vapor Pressure Increase With Temperature.
From mungfali.com
Water Vapor Pressure Temperature Chart Y Does Vapor Pressure Increase With Temperature as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the vapor pressure of a liquid varies with its temperature, as the following graph shows. Y Does Vapor Pressure Increase With Temperature.
From dxopxntvm.blob.core.windows.net
Why Does Water Vapor Pressure Increase With Temperature at Antonio Y Does Vapor Pressure Increase With Temperature generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. the increase in vapor pressure with temperature is not linear, however. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. as the temperature of a liquid increases, the vapor pressure of the liquid. Y Does Vapor Pressure Increase With Temperature.
From slideplayer.com
Aim How does temperature affect the vapor pressure of liquids Y Does Vapor Pressure Increase With Temperature the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. the increase in vapor pressure with temperature is not linear, however. The line on the. Y Does Vapor Pressure Increase With Temperature.
From schematicdiagramyakuza.z13.web.core.windows.net
Pressure Temperature Diagram Y Does Vapor Pressure Increase With Temperature as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. the vapor pressure of a liquid varies with its temperature, as the following graph shows for water. the increase in vapor pressure with temperature is not linear, however. in an open. Y Does Vapor Pressure Increase With Temperature.
From peoi.net
The vapor pressure of a liquid depends on the identity of the liquid Y Does Vapor Pressure Increase With Temperature the increase in vapor pressure with temperature is not linear, however. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. generally a substance's vapor pressure increases as temperature increases and decreases as temperature decreases (i.e. The line on the graph shows the boiling. the vapor pressure of a. Y Does Vapor Pressure Increase With Temperature.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Y Does Vapor Pressure Increase With Temperature as the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in. The line on the graph shows the boiling. in an open container, vapor pressure rises as temperature increases until the temperature reaches the boiling point. the vapor pressure of a liquid varies. Y Does Vapor Pressure Increase With Temperature.