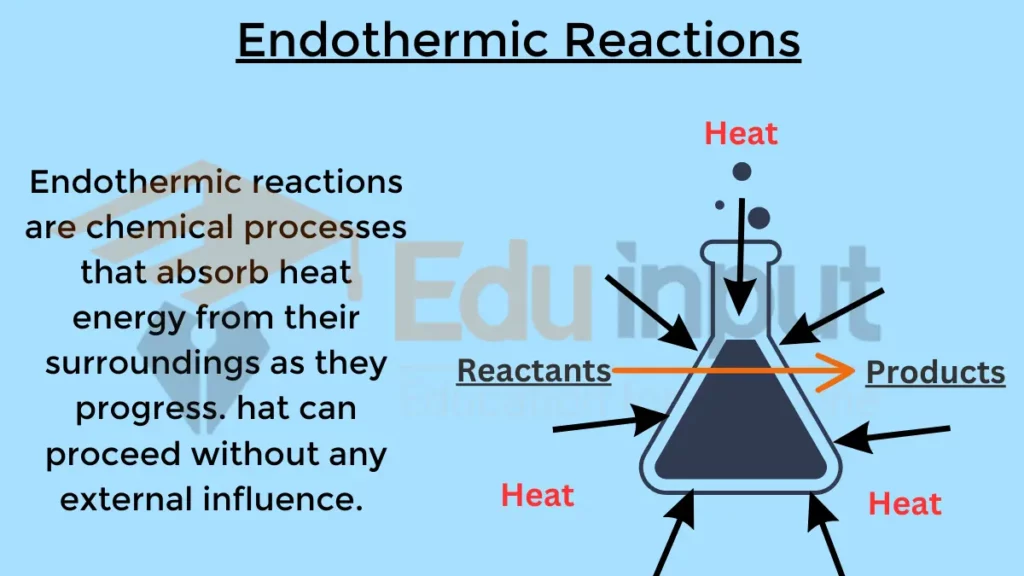
Clothes Drying Endothermic Exothermic . An example of evaporation is the drying of wet clothes. Determine if a chemical process is exothermic or endothermic. Define endothermic and exothermic reactions. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter q q. Drying cloth involves two processes. The sign of q q for an endothermic process is positive because the. How do clothes dry or even how does water in any place dry out without heating it up? Describe how heat is transferred in endothermic and exothermic reactions. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. Use or interpret energy diagrams. It’s obviously that water boils and evaporates but how does. In this case, the water droplets inside the clothes (system) pull the.
from eduinput.com
Use or interpret energy diagrams. Define endothermic and exothermic reactions. The sign of q q for an endothermic process is positive because the. The quantity of heat for a process is represented by the letter q q. Determine if a chemical process is exothermic or endothermic. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. An example of evaporation is the drying of wet clothes. Describe how heat is transferred in endothermic and exothermic reactions. It’s obviously that water boils and evaporates but how does. Drying cloth involves two processes.
Endothermic ReactionsCharacteristics, Identification, and Examples
Clothes Drying Endothermic Exothermic In this case, the water droplets inside the clothes (system) pull the. It’s obviously that water boils and evaporates but how does. The sign of q q for an endothermic process is positive because the. Use or interpret energy diagrams. In this case, the water droplets inside the clothes (system) pull the. An example of evaporation is the drying of wet clothes. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. Determine if a chemical process is exothermic or endothermic. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter q q. Define endothermic and exothermic reactions. How do clothes dry or even how does water in any place dry out without heating it up? Drying cloth involves two processes. Describe how heat is transferred in endothermic and exothermic reactions.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Clothes Drying Endothermic Exothermic Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. The sign of q q for an endothermic process is positive because the. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter q q. Determine if a chemical process. Clothes Drying Endothermic Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Clothes Drying Endothermic Exothermic It’s obviously that water boils and evaporates but how does. In this case, the water droplets inside the clothes (system) pull the. The sign of q q for an endothermic process is positive because the. Drying cloth involves two processes. Use or interpret energy diagrams. Energy has to be provided to change the water from liquid to vapour and an. Clothes Drying Endothermic Exothermic.
From studylib.net
Exothermic and Endothermic Reactions Lab Clothes Drying Endothermic Exothermic Use or interpret energy diagrams. It’s obviously that water boils and evaporates but how does. In this case, the water droplets inside the clothes (system) pull the. Define endothermic and exothermic reactions. How do clothes dry or even how does water in any place dry out without heating it up? The quantity of heat for a process is represented by. Clothes Drying Endothermic Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Clothes Drying Endothermic Exothermic Use or interpret energy diagrams. The quantity of heat for a process is represented by the letter q q. Drying cloth involves two processes. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. An example of evaporation is the drying of wet clothes. It’s obviously that. Clothes Drying Endothermic Exothermic.
From www.pinterest.ph
Endothermic and Exothermic Reactions infographic diagram showing Clothes Drying Endothermic Exothermic Drying cloth involves two processes. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter q q. The sign of q q for an endothermic process is positive because the. How do clothes dry or even how does water in any place dry out without heating it up? Use or interpret energy diagrams.. Clothes Drying Endothermic Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Clothes Drying Endothermic Exothermic The sign of q q for an endothermic process is positive because the. Drying cloth involves two processes. It’s obviously that water boils and evaporates but how does. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. Use or interpret energy diagrams. In this case, the. Clothes Drying Endothermic Exothermic.
From whatisdiffer.com
Difference Between Endothermic And Exothermic Reactions? Clothes Drying Endothermic Exothermic Define endothermic and exothermic reactions. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. The quantity of heat for a process is represented by the letter q q. Determine if a chemical process is exothermic or endothermic. Describe how heat is transferred in endothermic and exothermic. Clothes Drying Endothermic Exothermic.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Examples Clothes Drying Endothermic Exothermic It’s obviously that water boils and evaporates but how does. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter q q. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. An example of evaporation is the drying of. Clothes Drying Endothermic Exothermic.
From slideplayer.com
CHAPTER ppt download Clothes Drying Endothermic Exothermic Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter q q. Use or interpret energy diagrams. How do clothes dry or even how does water in any place dry. Clothes Drying Endothermic Exothermic.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Clothes Drying Endothermic Exothermic How do clothes dry or even how does water in any place dry out without heating it up? An example of evaporation is the drying of wet clothes. Determine if a chemical process is exothermic or endothermic. It’s obviously that water boils and evaporates but how does. Energy has to be provided to change the water from liquid to vapour. Clothes Drying Endothermic Exothermic.
From eduinput.com
Difference Between Endothermic And Exothermic Reactions Clothes Drying Endothermic Exothermic Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. Describe how heat is transferred in endothermic and exothermic reactions. The sign of q q for an endothermic process is positive because the. Determine if a chemical process is exothermic or endothermic. Define endothermic and exothermic reactions.. Clothes Drying Endothermic Exothermic.
From www.tes.com
Exothermic and Endothermic Reactions Teaching Resources Clothes Drying Endothermic Exothermic The quantity of heat for a process is represented by the letter q q. Define endothermic and exothermic reactions. Use or interpret energy diagrams. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. How do clothes dry or even how does water in any place dry. Clothes Drying Endothermic Exothermic.
From physicsinmyview.com
10 Examples of Endothermic Reactions in Everyday Life Physics In My View Clothes Drying Endothermic Exothermic Determine if a chemical process is exothermic or endothermic. Describe how heat is transferred in endothermic and exothermic reactions. Use or interpret energy diagrams. The sign of q q for an endothermic process is positive because the. It’s obviously that water boils and evaporates but how does. Energy has to be provided to change the water from liquid to vapour. Clothes Drying Endothermic Exothermic.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Clothes Drying Endothermic Exothermic It’s obviously that water boils and evaporates but how does. The quantity of heat for a process is represented by the letter q q. An example of evaporation is the drying of wet clothes. How do clothes dry or even how does water in any place dry out without heating it up? Determine if a chemical process is exothermic or. Clothes Drying Endothermic Exothermic.
From learningclignensembleu9.z22.web.core.windows.net
Endothermic And Exothermic Reaction Worksheet Clothes Drying Endothermic Exothermic The quantity of heat for a process is represented by the letter q q. How do clothes dry or even how does water in any place dry out without heating it up? It’s obviously that water boils and evaporates but how does. Energy has to be provided to change the water from liquid to vapour and an air stream is. Clothes Drying Endothermic Exothermic.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Clothes Drying Endothermic Exothermic Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. Determine if a chemical process is exothermic or endothermic. Drying cloth involves two processes. An example of evaporation is the drying of wet clothes. It’s obviously that water boils and evaporates but how does. Define endothermic and. Clothes Drying Endothermic Exothermic.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Clothes Drying Endothermic Exothermic Drying cloth involves two processes. The sign of q q for an endothermic process is positive because the. In this case, the water droplets inside the clothes (system) pull the. Use or interpret energy diagrams. How do clothes dry or even how does water in any place dry out without heating it up? Define endothermic and exothermic reactions. Describe how. Clothes Drying Endothermic Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Clothes Drying Endothermic Exothermic The quantity of heat for a process is represented by the letter q q. An example of evaporation is the drying of wet clothes. The sign of q q for an endothermic process is positive because the. Define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. It’s obviously that water boils and evaporates but. Clothes Drying Endothermic Exothermic.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Clothes Drying Endothermic Exothermic How do clothes dry or even how does water in any place dry out without heating it up? Define endothermic and exothermic reactions. Determine if a chemical process is exothermic or endothermic. It’s obviously that water boils and evaporates but how does. The sign of q q for an endothermic process is positive because the. Describe how heat is transferred. Clothes Drying Endothermic Exothermic.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Clothes Drying Endothermic Exothermic Use or interpret energy diagrams. The sign of q q for an endothermic process is positive because the. The quantity of heat for a process is represented by the letter q q. Drying cloth involves two processes. Define endothermic and exothermic reactions. In this case, the water droplets inside the clothes (system) pull the. Describe how heat is transferred in. Clothes Drying Endothermic Exothermic.
From mungfali.com
Exothermic Vs Endothermic Diagram Clothes Drying Endothermic Exothermic Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. Define endothermic and exothermic reactions. In this case, the water droplets inside the clothes (system) pull the. Use or interpret energy diagrams. The quantity of heat for a process is represented by the letter q q. It’s. Clothes Drying Endothermic Exothermic.
From slideplayer.com
Endothermic or Exothermic?? ppt download Clothes Drying Endothermic Exothermic The quantity of heat for a process is represented by the letter q q. Define endothermic and exothermic reactions. Describe how heat is transferred in endothermic and exothermic reactions. How do clothes dry or even how does water in any place dry out without heating it up? In this case, the water droplets inside the clothes (system) pull the. Determine. Clothes Drying Endothermic Exothermic.
From 7esl.com
Exothermic vs. Endothermic The Main Difference • 7ESL Clothes Drying Endothermic Exothermic Drying cloth involves two processes. It’s obviously that water boils and evaporates but how does. The quantity of heat for a process is represented by the letter q q. Use or interpret energy diagrams. Determine if a chemical process is exothermic or endothermic. In this case, the water droplets inside the clothes (system) pull the. The sign of q q. Clothes Drying Endothermic Exothermic.
From www.savemyexams.com
Endothermic & Exothermic Reactions Cambridge O Level Chemistry Clothes Drying Endothermic Exothermic Use or interpret energy diagrams. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. Determine if a chemical process is exothermic or endothermic. How do clothes dry or even how does water in any place dry out without heating it up? An example of evaporation is. Clothes Drying Endothermic Exothermic.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Clothes Drying Endothermic Exothermic Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. The sign of q q for an endothermic process is positive because the. In this case, the water droplets inside the clothes (system) pull the. Determine if a chemical process is exothermic or endothermic. Define endothermic and. Clothes Drying Endothermic Exothermic.
From 7esl.com
Exothermic vs. Endothermic The Main Difference • 7ESL Clothes Drying Endothermic Exothermic Describe how heat is transferred in endothermic and exothermic reactions. Drying cloth involves two processes. Use or interpret energy diagrams. Define endothermic and exothermic reactions. In this case, the water droplets inside the clothes (system) pull the. Define endothermic and exothermic reactions. The quantity of heat for a process is represented by the letter q q. How do clothes dry. Clothes Drying Endothermic Exothermic.
From www.slideserve.com
PPT Endothermic and exothermic reactions PowerPoint Presentation Clothes Drying Endothermic Exothermic Determine if a chemical process is exothermic or endothermic. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. How do clothes dry or even how does water in any place dry out without heating it up? Use or interpret energy diagrams. The quantity of heat for. Clothes Drying Endothermic Exothermic.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation Clothes Drying Endothermic Exothermic Define endothermic and exothermic reactions. Determine if a chemical process is exothermic or endothermic. In this case, the water droplets inside the clothes (system) pull the. It’s obviously that water boils and evaporates but how does. Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. An. Clothes Drying Endothermic Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Clothes Drying Endothermic Exothermic An example of evaporation is the drying of wet clothes. Use or interpret energy diagrams. Define endothermic and exothermic reactions. Determine if a chemical process is exothermic or endothermic. The quantity of heat for a process is represented by the letter q q. Define endothermic and exothermic reactions. Energy has to be provided to change the water from liquid to. Clothes Drying Endothermic Exothermic.
From www.difference101.com
Endothermic vs. Exothermic 5 Key Differences, Pros & Cons, Examples Clothes Drying Endothermic Exothermic Energy has to be provided to change the water from liquid to vapour and an air stream is needed to remove the vapour. An example of evaporation is the drying of wet clothes. The quantity of heat for a process is represented by the letter q q. Define endothermic and exothermic reactions. In this case, the water droplets inside the. Clothes Drying Endothermic Exothermic.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Clothes Drying Endothermic Exothermic It’s obviously that water boils and evaporates but how does. Define endothermic and exothermic reactions. Drying cloth involves two processes. The quantity of heat for a process is represented by the letter q q. Define endothermic and exothermic reactions. How do clothes dry or even how does water in any place dry out without heating it up? Energy has to. Clothes Drying Endothermic Exothermic.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Clothes Drying Endothermic Exothermic The quantity of heat for a process is represented by the letter q q. Drying cloth involves two processes. Use or interpret energy diagrams. It’s obviously that water boils and evaporates but how does. Describe how heat is transferred in endothermic and exothermic reactions. An example of evaporation is the drying of wet clothes. Energy has to be provided to. Clothes Drying Endothermic Exothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Clothes Drying Endothermic Exothermic Define endothermic and exothermic reactions. In this case, the water droplets inside the clothes (system) pull the. Describe how heat is transferred in endothermic and exothermic reactions. How do clothes dry or even how does water in any place dry out without heating it up? It’s obviously that water boils and evaporates but how does. Determine if a chemical process. Clothes Drying Endothermic Exothermic.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Clothes Drying Endothermic Exothermic It’s obviously that water boils and evaporates but how does. Define endothermic and exothermic reactions. The sign of q q for an endothermic process is positive because the. Define endothermic and exothermic reactions. How do clothes dry or even how does water in any place dry out without heating it up? Drying cloth involves two processes. In this case, the. Clothes Drying Endothermic Exothermic.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Clothes Drying Endothermic Exothermic In this case, the water droplets inside the clothes (system) pull the. Drying cloth involves two processes. Describe how heat is transferred in endothermic and exothermic reactions. Define endothermic and exothermic reactions. The sign of q q for an endothermic process is positive because the. The quantity of heat for a process is represented by the letter q q. Use. Clothes Drying Endothermic Exothermic.