Magnesium Sulfate Van't Hoff . The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. 2) solve for the osmotic pressure: For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. 1) calculate the van 't hoff factor from the degree of dissociation: It shows that a plot. The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. It shows that a plot of \(\ln k\) vs. Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\).
from www.numerade.com
Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. 2) solve for the osmotic pressure: Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). 1) calculate the van 't hoff factor from the degree of dissociation: Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. It shows that a plot. It shows that a plot of \(\ln k\) vs. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2.
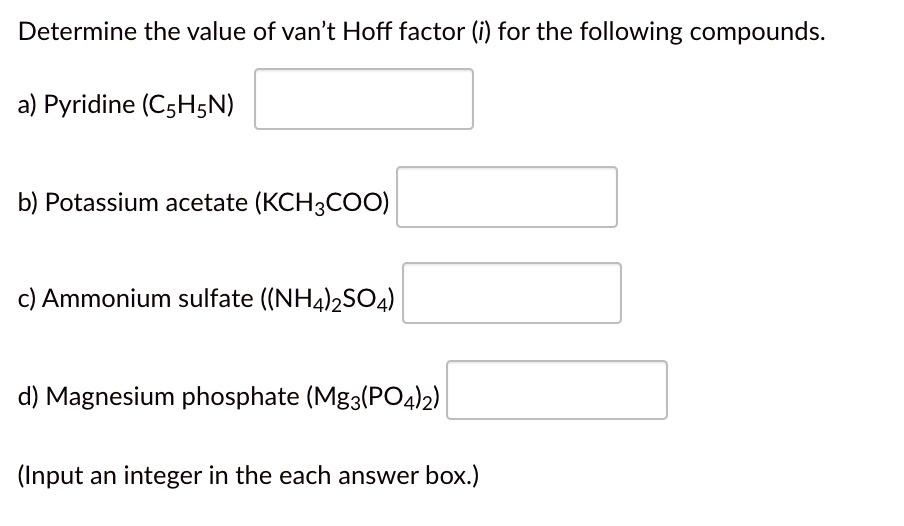
SOLVED Determine the value of van't Hoff factor (i) for the following
Magnesium Sulfate Van't Hoff Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. It shows that a plot of \(\ln k\) vs. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. It shows that a plot. For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). 1) calculate the van 't hoff factor from the degree of dissociation: Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. 2) solve for the osmotic pressure:
From www.newlandfert.com
Magnesium Sulphate Monohydrate (Kieserite) GranularNewland Resources Ltd Magnesium Sulfate Van't Hoff The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. 2) solve for the osmotic. Magnesium Sulfate Van't Hoff.
From brainly.in
what would be the value of Van"t Hoff factor for dilute aqueous Magnesium Sulfate Van't Hoff Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). 1) calculate the van 't hoff factor from the degree. Magnesium Sulfate Van't Hoff.
From educationgrafts.z21.web.core.windows.net
What Is Van't Hoff Factor Shaalaa Magnesium Sulfate Van't Hoff The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. It shows that a plot. Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). It shows that a plot of \(\ln k\) vs. Α = 0.80 n = 2 i = 1 + 0.80(2 − 1). Magnesium Sulfate Van't Hoff.
From www.numerade.com
SOLVED 4iSee Periodic Table See Hint The van't Hoff factor for a 0. Magnesium Sulfate Van't Hoff Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. It shows that a plot of \(\ln k\) vs. Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. The theoretical van’t. Magnesium Sulfate Van't Hoff.
From syndel.com
Sodium Thiosulfate for Hatcheries and Aquaculture Facilities Syndel Magnesium Sulfate Van't Hoff Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. 2) solve for the osmotic pressure: It shows that a plot of \(\ln k\) vs. For example, if a compound fully dissociates into two ions, the van’t. Magnesium Sulfate Van't Hoff.
From www.vaniperen.com
Magnesium Sulphate Van Iperen International Magnesium Sulfate Van't Hoff Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). It shows that a plot. 1) calculate the van 't hoff factor from the degree of dissociation: For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i. Magnesium Sulfate Van't Hoff.
From zhuanlan.zhihu.com
镁科研:镁基储氢合金的吸放氢反应热力学和动力学 知乎 Magnesium Sulfate Van't Hoff The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. It shows that a plot. 1) calculate the van 't hoff factor from the degree of dissociation: Notably, the apelblat model was found to be more accurate and reliable in. Magnesium Sulfate Van't Hoff.
From blog.websoft9.com
How To Find Van T Hoff Factor Hot Sale Magnesium Sulfate Van't Hoff Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). Notably, the apelblat model was found to be more accurate and. Magnesium Sulfate Van't Hoff.
From www.numerade.com
SOLVED Using the van't Hoff factors in Table 12.7, calculate the mass Magnesium Sulfate Van't Hoff For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. It shows that a plot of \(\ln k\) vs. 1) calculate the van 't hoff factor from the degree of dissociation: Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). Α = 0.80 n = 2 i = 1 +. Magnesium Sulfate Van't Hoff.
From www.chegg.com
Solved A Student Conducts An Experiment To Determine The Magnesium Sulfate Van't Hoff It shows that a plot. It shows that a plot of \(\ln k\) vs. 2) solve for the osmotic pressure: The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. For example, if a compound fully dissociates into two ions,. Magnesium Sulfate Van't Hoff.
From www.numerade.com
SOLVED Use the van't Hoff factors in Table 13.9 to calculate each Magnesium Sulfate Van't Hoff Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. It shows that a plot of \(\ln k\) vs. 1) calculate the van 't hoff factor from the degree of dissociation: Calculate the van’t. Magnesium Sulfate Van't Hoff.
From www.slideserve.com
PPT Chapter 14 Solutions and Their Properties If you’re not part of Magnesium Sulfate Van't Hoff It shows that a plot of \(\ln k\) vs. Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). 2) solve for. Magnesium Sulfate Van't Hoff.
From gossipvehiculo.com
Biografía Quien era Dilano van 't Hoff el Aspirante a Fórmula 1 Cuyo Magnesium Sulfate Van't Hoff Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. 1) calculate the van 't hoff factor from the degree of dissociation: For example, if a compound fully dissociates into two ions, the. Magnesium Sulfate Van't Hoff.
From www.researchgate.net
Van't Hoff plots of some selected hydrides. The stabilization of the Magnesium Sulfate Van't Hoff It shows that a plot of \(\ln k\) vs. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. 2) solve for the osmotic pressure: It shows that a plot. The van’t hoff factor. Magnesium Sulfate Van't Hoff.
From educationgrafts.z21.web.core.windows.net
Vant Hoff Factor For C6h12o6 Magnesium Sulfate Van't Hoff It shows that a plot of \(\ln k\) vs. Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. 2) solve for the osmotic pressure: For example, if a compound fully dissociates into two. Magnesium Sulfate Van't Hoff.
From www.showme.com
Van’t Hoff’s Factor Science ShowMe Magnesium Sulfate Van't Hoff The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. 2) solve for the osmotic pressure: 1) calculate the van 't hoff factor from the degree of dissociation: It shows that a plot of \(\ln k\) vs. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a. Magnesium Sulfate Van't Hoff.
From chemistnotes.com
Osmosis/Reverse osmosis/osmotic pressure/Definition/Equation Magnesium Sulfate Van't Hoff It shows that a plot. Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. 2) solve for the osmotic. Magnesium Sulfate Van't Hoff.
From www.numerade.com
SOLVEDThe van't Hoff factor (i), is a measure of association or Magnesium Sulfate Van't Hoff Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. It shows that a plot of \(\ln k\) vs. 1) calculate the van 't hoff factor from the degree of dissociation: The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in. Magnesium Sulfate Van't Hoff.
From www.numerade.com
SOLVED Determine the value of van't Hoff factor (i) for the following Magnesium Sulfate Van't Hoff For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. 1) calculate the van 't hoff factor from the degree of dissociation: Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a. Magnesium Sulfate Van't Hoff.
From www.youtube.com
Finding the ideal Van't Hoff factor YouTube Magnesium Sulfate Van't Hoff Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. It shows that a plot. 1) calculate the van 't hoff factor from the degree of dissociation: 2) solve for the osmotic pressure: It shows that a plot of \(\ln k\) vs. Notably, the apelblat model was found to be more accurate and reliable. Magnesium Sulfate Van't Hoff.
From www.chegg.com
Solved I need help finding the van't hoff factor for MgCl2. Magnesium Sulfate Van't Hoff Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. It shows that a plot. 1) calculate the van 't hoff factor from the degree of dissociation: For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2.. Magnesium Sulfate Van't Hoff.
From animalia-life.club
Magnesium Sulfate Lewis Structure Magnesium Sulfate Van't Hoff Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. 1) calculate the van 't hoff factor from. Magnesium Sulfate Van't Hoff.
From wendymaxwell196news.blogspot.com
Van T Hoff Equation Calculator Magnesium Sulfate Van't Hoff 2) solve for the osmotic pressure: 1) calculate the van 't hoff factor from the degree of dissociation: Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. It shows that a plot.. Magnesium Sulfate Van't Hoff.
From www.numerade.com
SOLVEDUse the van't Hoff factors in Table 14.9 to calculate each Magnesium Sulfate Van't Hoff The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. It shows that a plot. The theoretical van’t hoff factor (i) represents the idealized. Magnesium Sulfate Van't Hoff.
From www.bartleby.com
Answered Use the MEASURED Van't Hoff factors in… bartleby Magnesium Sulfate Van't Hoff It shows that a plot of \(\ln k\) vs. For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. It shows that a plot. Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. 1) calculate the van 't hoff factor from the. Magnesium Sulfate Van't Hoff.
From www.youtube.com
Derive the Van’t Hoff Equation sweet and easy using the Gibbs Magnesium Sulfate Van't Hoff It shows that a plot. 2) solve for the osmotic pressure: It shows that a plot of \(\ln k\) vs. Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a. Magnesium Sulfate Van't Hoff.
From www.numerade.com
SOLVEDReferring to the van"t Hoff factors in Table 13.7, calculate the Magnesium Sulfate Van't Hoff The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. 1) calculate the van 't hoff factor from the degree of dissociation: Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. Α. Magnesium Sulfate Van't Hoff.
From www.chegg.com
Solved Exercise 14.87 Use the van't Hoff factors in the Magnesium Sulfate Van't Hoff It shows that a plot of \(\ln k\) vs. It shows that a plot. The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate. Magnesium Sulfate Van't Hoff.
From www.pngegg.com
Sulfato de magnesio lewis estructura anhidra, sulfato de sodio, ángulo Magnesium Sulfate Van't Hoff 2) solve for the osmotic pressure: For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. It shows that a plot. Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. 1) calculate the van. Magnesium Sulfate Van't Hoff.
From www.numerade.com
SOLVED Referring to the van't Hoff factors in the table below Magnesium Sulfate Van't Hoff For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. It shows that a plot. 2) solve for the osmotic pressure: The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. 1) calculate the van 't hoff factor from the degree of dissociation: It. Magnesium Sulfate Van't Hoff.
From www.chegg.com
Solved Using The Van’t Hoff Factors In The Table Below, C... Magnesium Sulfate Van't Hoff It shows that a plot. 1) calculate the van 't hoff factor from the degree of dissociation: It shows that a plot of \(\ln k\) vs. For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in. Magnesium Sulfate Van't Hoff.
From www.youtube.com
Van't Hoff Equation Exam Problems! (Thermodynamics) YouTube Magnesium Sulfate Van't Hoff It shows that a plot of \(\ln k\) vs. 1) calculate the van 't hoff factor from the degree of dissociation: The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate into in a solution. For example, if a compound. Magnesium Sulfate Van't Hoff.
From www.elgencurioso.com
Factor de van't Hoff Definición y cómo calcularlo El Gen Curioso Magnesium Sulfate Van't Hoff Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). For example, if a compound fully dissociates into two ions, the van’t hoff factor is 2. Α = 0.80 n = 2 i = 1 + 0.80(2 − 1) i = 1.80. It shows that a plot. The van’t hoff factor explains how many ions are produced. Magnesium Sulfate Van't Hoff.
From pantip.com
รบกวนผู้รู้สอนทำ โจทย์เคมีฟิสิกส์ 2 ข้อนี้หน่อยครับ ขอบคุณครับผม Pantip Magnesium Sulfate Van't Hoff It shows that a plot. 1) calculate the van 't hoff factor from the degree of dissociation: 2) solve for the osmotic pressure: Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol. The theoretical van’t hoff factor (i) represents the idealized number of particles a solute should dissociate. Magnesium Sulfate Van't Hoff.
From www.chegg.com
Solved Using the van’t Hoff factors in the table below, Magnesium Sulfate Van't Hoff 1) calculate the van 't hoff factor from the degree of dissociation: Calculate the van’t hoff factor for a 0.050 m aqueous solution of \(mgcl_2\). The van’t hoff factor explains how many ions are produced when an electrolyte dissolves. Notably, the apelblat model was found to be more accurate and reliable in correlating the solubility of mgso 4 in ethanol.. Magnesium Sulfate Van't Hoff.