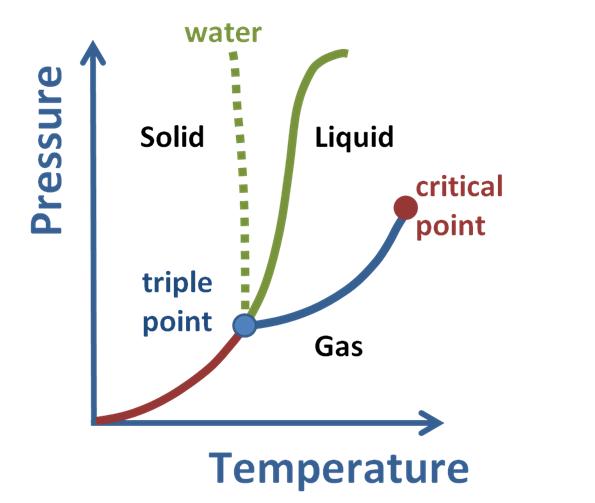
Temperature At Which Solid And Liquid Coexist . The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. The state that a given substance exhibits is also a. The temperature at which solid and liquid states coexist together is called the melting point. The point where the lines intersect is called the triple point. The triple point is the one condition of. Quartz (solid), water (liquid), nitrogen dioxide (gas). 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas.
from www.coursehero.com
The state that a given substance exhibits is also a. The triple point is the one condition of. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. The point where the lines intersect is called the triple point. The temperature at which solid and liquid states coexist together is called the melting point. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. Quartz (solid), water (liquid), nitrogen dioxide (gas). The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid.
Solid to Gas Phase Transition Introduction to Chemistry Course Hero
Temperature At Which Solid And Liquid Coexist The triple point is the one condition of. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. The triple point is the one condition of. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The point where the lines intersect is called the triple point. The state that a given substance exhibits is also a. Quartz (solid), water (liquid), nitrogen dioxide (gas). The temperature at which solid and liquid states coexist together is called the melting point.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Temperature At Which Solid And Liquid Coexist The point where the lines intersect is called the triple point. The temperature at which solid and liquid states coexist together is called the melting point. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. It is the pressure and temperature conditions at which all. Temperature At Which Solid And Liquid Coexist.
From x-engineer.org
Which are the states of matter Temperature At Which Solid And Liquid Coexist A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. Quartz (solid), water (liquid), nitrogen. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Phase Changes. ppt download Temperature At Which Solid And Liquid Coexist The temperature at which solid and liquid states coexist together is called the melting point. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. The triple point is the one condition of. The melting point of a substance is the temperature at which a solid and liquid phase may coexist. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Liquids and Solids Changes of State. ppt video online download Temperature At Which Solid And Liquid Coexist 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The temperature at which solid and liquid states coexist together is called the melting point. The triple point is the one condition of. The melting point of a substance is the temperature at which a solid. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Phases of Matter. ppt download Temperature At Which Solid And Liquid Coexist The point where the lines intersect is called the triple point. Quartz (solid), water (liquid), nitrogen dioxide (gas). The temperature at which solid and liquid states coexist together is called the melting point. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. It is the. Temperature At Which Solid And Liquid Coexist.
From www.youtube.com
At what temperature does solid and liquid coexist? YouTube Temperature At Which Solid And Liquid Coexist The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The temperature at which solid. Temperature At Which Solid And Liquid Coexist.
From www.numerade.com
SOLVED 1. Pressure on the surface of a gas is increased. What will Temperature At Which Solid And Liquid Coexist The triple point is the one condition of. The temperature at which solid and liquid states coexist together is called the melting point. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. The point where the lines intersect is called the triple point. Quartz (solid), water (liquid), nitrogen dioxide (gas).. Temperature At Which Solid And Liquid Coexist.
From invaderxan.tumblr.com
Mostly Harmless — Phase diagram for water. The triple point is the... Temperature At Which Solid And Liquid Coexist A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The point where the lines intersect is called the triple point. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. The temperature at which solid and. Temperature At Which Solid And Liquid Coexist.
From www.researchgate.net
Instantaneous (a) temperature and (b) pressure of four solidliquid Temperature At Which Solid And Liquid Coexist The point where the lines intersect is called the triple point. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. The state that a given substance exhibits is also a. A phase diagram is a graph which the. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Liquids Chapter ppt download Temperature At Which Solid And Liquid Coexist The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. The point where the lines intersect is called the triple point. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Colligative Properties of Solutions ppt download Temperature At Which Solid And Liquid Coexist Quartz (solid), water (liquid), nitrogen dioxide (gas). The state that a given substance exhibits is also a. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. A phase diagram is a graph which the conditions of temperature and. Temperature At Which Solid And Liquid Coexist.
From www.numerade.com
SOLVED atm) pressure H temperature (K) Which is the solid onephase Temperature At Which Solid And Liquid Coexist The state that a given substance exhibits is also a. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The triple point is the one condition. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Temperature, Heat, and the First Law of Thermodynamics ppt download Temperature At Which Solid And Liquid Coexist The triple point is the one condition of. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. The point where the lines intersect is called the. Temperature At Which Solid And Liquid Coexist.
From naeye.net
Triple Point of Water The Temperature Where All Three Phases Coexist Temperature At Which Solid And Liquid Coexist 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. It is the pressure and temperature conditions at which all three phases,. Temperature At Which Solid And Liquid Coexist.
From www.gauthmath.com
Solved The graph shows the phase diagram of a substance. At which Temperature At Which Solid And Liquid Coexist The triple point is the one condition of. The temperature at which solid and liquid states coexist together is called the melting point. The point where the lines intersect is called the triple point. Quartz (solid), water (liquid), nitrogen dioxide (gas). It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium.. Temperature At Which Solid And Liquid Coexist.
From www.chegg.com
Solved 3. A liquid and a solid of the same substance coexist Temperature At Which Solid And Liquid Coexist It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. The state that a given substance exhibits is also a.. Temperature At Which Solid And Liquid Coexist.
From www.researchgate.net
Equilibrium coexistence of ice and water. Illustration of potential Temperature At Which Solid And Liquid Coexist The state that a given substance exhibits is also a. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The temperature at which solid and liquid states coexist together is called the melting point. The triple point is the one condition of. It is the. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Gases and States of Matter Unit 8 ppt download Temperature At Which Solid And Liquid Coexist The triple point is the one condition of. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas,. Temperature At Which Solid And Liquid Coexist.
From www.numerade.com
SOLVED 'Phase Diagram L Temperature The graph shows the phase diagram Temperature At Which Solid And Liquid Coexist The state that a given substance exhibits is also a. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. The triple point is the one condition of. The temperature at which solid and liquid states coexist together is called the melting point. The point where the lines intersect is called. Temperature At Which Solid And Liquid Coexist.
From www.researchgate.net
Phase transitions with triple points. a, The phase diagram of water Temperature At Which Solid And Liquid Coexist It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The triple point is the one condition of. The melting point of a substance is the temperature. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Acid, Bases and Water. ppt download Temperature At Which Solid And Liquid Coexist It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). Quartz (solid), water (liquid), nitrogen dioxide (gas). The temperature at which solid and liquid states coexist together. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Liquids and Solids Chapter ppt download Temperature At Which Solid And Liquid Coexist The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. Quartz (solid), water (liquid), nitrogen dioxide (gas). The state that a given substance exhibits is also a. It is the pressure and temperature conditions at which all three phases,. Temperature At Which Solid And Liquid Coexist.
From www.coursehero.com
Solid to Gas Phase Transition Introduction to Chemistry Course Hero Temperature At Which Solid And Liquid Coexist The triple point is the one condition of. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. The temperature at which solid and liquid states coexist together is called the melting point. The point where the lines intersect. Temperature At Which Solid And Liquid Coexist.
From chem.libretexts.org
8.4 Coexistence Curves Chemistry LibreTexts Temperature At Which Solid And Liquid Coexist The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. Quartz (solid), water (liquid), nitrogen dioxide (gas). The triple point is the one condition of. The state that a given substance exhibits is also a. The point where the. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Phase Changes and Intermolecular Forces ppt download Temperature At Which Solid And Liquid Coexist The triple point is the one condition of. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The state that a given substance exhibits is also a. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in. Temperature At Which Solid And Liquid Coexist.
From samson-jolpblogsantos.blogspot.com
How Does Temperature Affect Solids Liquids and Gases Temperature At Which Solid And Liquid Coexist It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which matter changes from solid to liquid. A phase diagram is a graph which the conditions of. Temperature At Which Solid And Liquid Coexist.
From www.numerade.com
SOLVED The below is the pressure temperature phase diagram of Hzo Temperature At Which Solid And Liquid Coexist A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The point where the lines intersect is called the triple point. The temperature at which solid and liquid states coexist together is called the melting point. The melting point of a substance is the temperature at. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Thermal & Lecture ppt download Temperature At Which Solid And Liquid Coexist The triple point is the one condition of. The point where the lines intersect is called the triple point. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). Quartz (solid), water (liquid), nitrogen dioxide (gas). The temperature at which solid and liquid states coexist together. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Phase Changes. ppt download Temperature At Which Solid And Liquid Coexist A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. The point where the lines intersect is called the triple point. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at which. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
MATTER & ITS PROPERTIES NOTESHEET ppt download Temperature At Which Solid And Liquid Coexist Quartz (solid), water (liquid), nitrogen dioxide (gas). The triple point is the one condition of. The point where the lines intersect is called the triple point. The temperature at which solid and liquid states coexist together is called the melting point. The state that a given substance exhibits is also a. It is the pressure and temperature conditions at which. Temperature At Which Solid And Liquid Coexist.
From www.chegg.com
Solved The segment where molecules in the liquid state are Temperature At Which Solid And Liquid Coexist The state that a given substance exhibits is also a. The point where the lines intersect is called the triple point. The temperature at which solid and liquid states coexist together is called the melting point. The melting point of a substance is the temperature at which a solid and liquid phase may coexist in equilibrium and the temperature at. Temperature At Which Solid And Liquid Coexist.
From unistudium.unipg.it
Phase Diagrams Temperature At Which Solid And Liquid Coexist 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). Quartz (solid), water (liquid), nitrogen dioxide (gas). The triple point is the one condition of. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. The melting. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
CHAPTER 12 LIQUIDS and SOLIDS ppt download Temperature At Which Solid And Liquid Coexist The triple point is the one condition of. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The point where the. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
Intermolecular Forces and ppt download Temperature At Which Solid And Liquid Coexist The point where the lines intersect is called the triple point. A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. It is the pressure and temperature conditions at which all three phases, solid, liquid, and gas, coexist in equilibrium. Quartz (solid), water (liquid), nitrogen dioxide. Temperature At Which Solid And Liquid Coexist.
From slideplayer.com
States and Changes of Matter ppt download Temperature At Which Solid And Liquid Coexist 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). A phase diagram is a graph which the conditions of temperature and pressure under which a substance exists in the solid, liquid, and gas. It is the pressure and temperature conditions at which all three phases,. Temperature At Which Solid And Liquid Coexist.