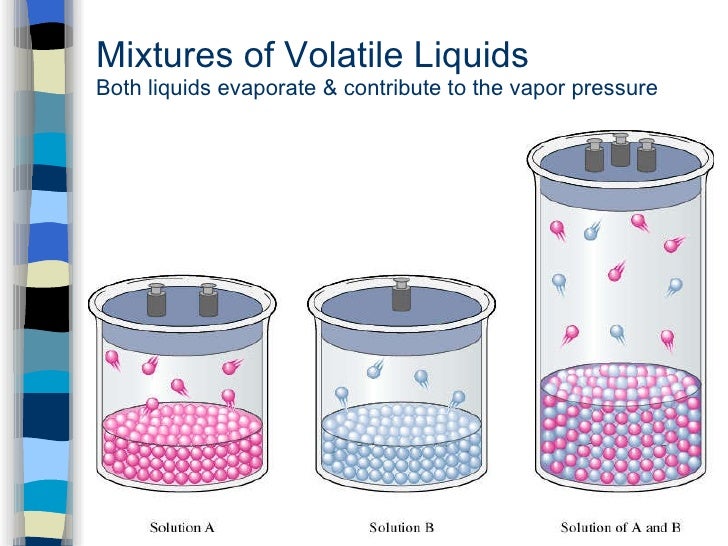
Volatile Solid Liquid Examples . A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. In volatile chemistry, volatility expresses the ability of a substance to vaporize. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Liquid mercury had a high vapor pressure, readily. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. Some solvents, such as acetone or ether, are particularly volatile. Volatility is not measured directly, but a. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. The composition of an ideal mixture containing at. Mercury is a volatile element. A substance with a high vapor pressure is said to be volatile.
from www.slideshare.net
Volatility is not measured directly, but a. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. A substance with a high vapor pressure is said to be volatile. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. Liquid mercury had a high vapor pressure, readily. Some solvents, such as acetone or ether, are particularly volatile. A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). In volatile chemistry, volatility expresses the ability of a substance to vaporize.
Solutions Powerpoint
Volatile Solid Liquid Examples Some solvents, such as acetone or ether, are particularly volatile. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). The composition of an ideal mixture containing at. Volatility is not measured directly, but a. Liquid mercury had a high vapor pressure, readily. A substance with a high vapor pressure is said to be volatile. Mercury is a volatile element. A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Some solvents, such as acetone or ether, are particularly volatile. The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. In volatile chemistry, volatility expresses the ability of a substance to vaporize.
From www.slideserve.com
PPT Solution Properties PowerPoint Presentation, free download ID7018658 Volatile Solid Liquid Examples A substance with a high vapor pressure is said to be volatile. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is not measured directly, but a. In volatile chemistry, volatility expresses the ability of a substance to vaporize. Examples of volatile substances include gasoline and rubbing alcohol. Volatile Solid Liquid Examples.
From worldnewlive.com
Which Is A Volatile Liquid? Mastery Wiki Volatile Solid Liquid Examples Liquid mercury had a high vapor pressure, readily. The composition of an ideal mixture containing at. Volatility is not measured directly, but a. A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. A substance with a high vapor pressure is said to be volatile. Examples of volatile substances. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT Anaerobic Digestion and Biogas PowerPoint Presentation, free download ID6070163 Volatile Solid Liquid Examples Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Liquid mercury had a high vapor pressure, readily. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. In volatile chemistry, volatility expresses the ability of a substance to vaporize. Examples of volatile substances include. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2392673 Volatile Solid Liquid Examples In volatile chemistry, volatility expresses the ability of a substance to vaporize. The composition of an ideal mixture containing at. Some solvents, such as acetone or ether, are particularly volatile. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. Volatility is a measure of how easily a material transitions into the gas phase via. Volatile Solid Liquid Examples.
From ar.inspiredpencil.com
Volatile Liquid Example Volatile Solid Liquid Examples Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a. Volatile Solid Liquid Examples.
From www.youtube.com
Properties of Solids, Liquids and Gases YouTube Volatile Solid Liquid Examples Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a. Volatile Solid Liquid Examples.
From www.youtube.com
Type detection for liquid mixture Volatile liquidsolid, VolatileNon volatile liquid YouTube Volatile Solid Liquid Examples A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. The composition of an ideal mixture containing at. Volatility is not measured directly, but a. Some solvents, such as acetone or ether, are particularly volatile. Liquid mercury had a high vapor pressure, readily. A substance with a high vapor pressure is said to be volatile.. Volatile Solid Liquid Examples.
From www.tutorix.com
Give three characteristics of solid liquid and gas Tutorix Volatile Solid Liquid Examples The composition of an ideal mixture containing at. A substance with a high vapor pressure is said to be volatile. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. In volatile chemistry, volatility expresses the ability of a substance to vaporize. Mercury is a volatile element. A volatile substance is any solid or liquid. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT Anaerobic Digestion and Biogas PowerPoint Presentation, free download ID6675248 Volatile Solid Liquid Examples Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Mercury is a volatile element. A substance with a high vapor pressure is said to be volatile. The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without. Volatile Solid Liquid Examples.
From ar.inspiredpencil.com
Volatile Liquid Volatile Solid Liquid Examples In volatile chemistry, volatility expresses the ability of a substance to vaporize. The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). Volatility is a measure of how easily. Volatile Solid Liquid Examples.
From ar.inspiredpencil.com
Volatile Liquid Example Volatile Solid Liquid Examples Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). In volatile chemistry, volatility expresses the ability of a substance to vaporize. Some solvents, such as acetone or ether, are particularly volatile. Liquid mercury had a high vapor pressure, readily. The term applies above all to liquids, but it can. Volatile Solid Liquid Examples.
From circuitengineverbis77.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram Volatile Solid Liquid Examples Liquid mercury had a high vapor pressure, readily. A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. Mercury is a volatile element. The composition of an ideal mixture containing at. A substance with a high vapor pressure is said to be volatile. Some solvents, such as acetone or. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT 4.5 Physical Properties of Covalent Molecules PowerPoint Presentation ID5675190 Volatile Solid Liquid Examples Mercury is a volatile element. In volatile chemistry, volatility expresses the ability of a substance to vaporize. Liquid mercury had a high vapor pressure, readily. Volatility is not measured directly, but a. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Some solvents, such as acetone or ether, are. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT Analysis for Total Solids PowerPoint Presentation, free download ID4475610 Volatile Solid Liquid Examples The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT Turbidity & Solids PowerPoint Presentation, free download ID6809595 Volatile Solid Liquid Examples Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. Some solvents, such as acetone or ether, are particularly volatile. Liquid mercury had a high vapor pressure, readily. The. Volatile Solid Liquid Examples.
From www.youtube.com
Separation of Volatile liquid solid mixture by Distillation; BSc Organic Chemistry Practical Volatile Solid Liquid Examples A substance with a high vapor pressure is said to be volatile. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. Volatility is not measured directly, but a. In volatile chemistry, volatility expresses the ability. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID2249941 Volatile Solid Liquid Examples A substance with a high vapor pressure is said to be volatile. A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. Mercury is a volatile element. Volatility is not measured directly, but a. Some solvents, such as acetone or ether, are particularly volatile. Volatility is a measure of. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT Chapter 10 Liquids and Solids PowerPoint Presentation, free download ID5888387 Volatile Solid Liquid Examples Mercury is a volatile element. In volatile chemistry, volatility expresses the ability of a substance to vaporize. Some solvents, such as acetone or ether, are particularly volatile. The composition of an ideal mixture containing at. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. The term applies above all to liquids, but it can. Volatile Solid Liquid Examples.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different states of Matters for Volatile Solid Liquid Examples In volatile chemistry, volatility expresses the ability of a substance to vaporize. Volatility is not measured directly, but a. Mercury is a volatile element. Liquid mercury had a high vapor pressure, readily. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). A volatile liquid is one that has an appreciable vapor pressure at the specified temperature.. Volatile Solid Liquid Examples.
From www.ase.org.uk
Solids, liquids and gases Volatile Solid Liquid Examples The composition of an ideal mixture containing at. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. Volatility is a measure of how easily a material. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT Solids Analysis PowerPoint Presentation, free download ID1820972 Volatile Solid Liquid Examples Some solvents, such as acetone or ether, are particularly volatile. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). A substance with a high vapor pressure is said to be volatile. A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so. Volatile Solid Liquid Examples.
From www.slideshare.net
Solutions Powerpoint Volatile Solid Liquid Examples The composition of an ideal mixture containing at. A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT SEWAGE CHARACTERISTICS PowerPoint Presentation ID5059312 Volatile Solid Liquid Examples Liquid mercury had a high vapor pressure, readily. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). Volatility is not measured directly, but a. The composition of an ideal mixture containing at. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. The term applies above all to liquids, but it. Volatile Solid Liquid Examples.
From www.chegg.com
Solved Question 4 Volatile Solids (VS) are a) Material Volatile Solid Liquid Examples Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene. Volatile Solid Liquid Examples.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma Volatile Solid Liquid Examples Liquid mercury had a high vapor pressure, readily. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). A substance with a high vapor pressure is said to be volatile. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). The term applies above all to liquids,. Volatile Solid Liquid Examples.
From sciencenotes.org
Volatility Volatile Definition in Chemistry Volatile Solid Liquid Examples In volatile chemistry, volatility expresses the ability of a substance to vaporize. The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. Examples of volatile substances include. Volatile Solid Liquid Examples.
From brainly.ph
Enrichment Activity 2 Drawing Solids, Liquids, la Caser Direction Draw five examples of solids Volatile Solid Liquid Examples A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. A substance with a high vapor pressure is said to be volatile. Liquid mercury had a high vapor pressure, readily. Mercury is a volatile. Volatile Solid Liquid Examples.
From saylordotorg.github.io
Solids, Liquids, and Gases Volatile Solid Liquid Examples The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). Volatility is not measured directly, but a. Examples of volatile substances include. Volatile Solid Liquid Examples.
From www.youtube.com
Examples of Solids Liquids and Gases 10 20 Examples of Solids Liquids and Gases in English Volatile Solid Liquid Examples A substance with a high vapor pressure is said to be volatile. Volatility is not measured directly, but a. The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. Liquid mercury had a high vapor pressure, readily. Volatility is a measure of. Volatile Solid Liquid Examples.
From www.pinterest.com
Liquid Definition Examples of Liquids Solid liquid gas, States of matter, Solutions and mixtures Volatile Solid Liquid Examples The term applies above all to liquids, but it can also relate to solids capable of passing directly to the vapour state, without passing through a liquid phase. A substance with a high vapor pressure is said to be volatile. Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). A volatile substance is any solid or. Volatile Solid Liquid Examples.
From www.thoughtco.com
Chemistry Glossary Definition of Volatile Volatile Solid Liquid Examples Mercury is a volatile element. In volatile chemistry, volatility expresses the ability of a substance to vaporize. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). The composition of an ideal mixture containing at. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature.. Volatile Solid Liquid Examples.
From www.slideserve.com
PPT Volatile Substances PowerPoint Presentation, free download ID1429973 Volatile Solid Liquid Examples Liquid mercury had a high vapor pressure, readily. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. Mercury is a volatile element. In volatile chemistry, volatility expresses the ability of a substance to vaporize. Some solvents, such as acetone or ether, are particularly volatile. The term applies above all to liquids, but it can. Volatile Solid Liquid Examples.
From www.youtube.com
10 Examples of Solid Liquid and Gas in English YouTube Volatile Solid Liquid Examples Examples of volatile substances include gasoline and rubbing alcohol (liquids) and paradichlorobenzene (solid). Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids) or sublimation (solids). A substance with a high vapor pressure is said to be volatile. Volatility is not measured directly, but a. The term applies above all to liquids, but. Volatile Solid Liquid Examples.
From www.studypool.com
SOLUTION Examples of solid liquid gas Studypool Volatile Solid Liquid Examples A volatile substance is any solid or liquid that has a high vapor pressure at room temperature, so it evaporates quickly. In volatile chemistry, volatility expresses the ability of a substance to vaporize. Liquid mercury had a high vapor pressure, readily. Mercury is a volatile element. Volatility is a measure of how easily a material transitions into the gas phase. Volatile Solid Liquid Examples.
From pediaa.com
Difference Between Volatile and Nonvolatile Substances Definition, Properties, Characteristics Volatile Solid Liquid Examples Liquid mercury had a high vapor pressure, readily. The composition of an ideal mixture containing at. A volatile liquid is one that has an appreciable vapor pressure at the specified temperature. A substance with a high vapor pressure is said to be volatile. Volatility is a measure of how easily a material transitions into the gas phase via evaporation (liquids). Volatile Solid Liquid Examples.