Avogadro's Number Standard Form . These particles could be electrons or molecules or atoms. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. How to use the ideal gas law. Overview of how avogadro's number is used to measure the number of units of any substance. accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. What is the molar mass formula? The units may be electrons, atoms, ions, or molecules,. avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. Therefore, the terms for the specific types of fundamental chemical particles, namely . one mole of a substance is equal to 6.022 × 10²³ units of that substance (such. In this form, it is properly known as avogadro's. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. At least it's not as hard to. what is avogadro’s number? avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement.
from printablelessontokimi88.z13.web.core.windows.net
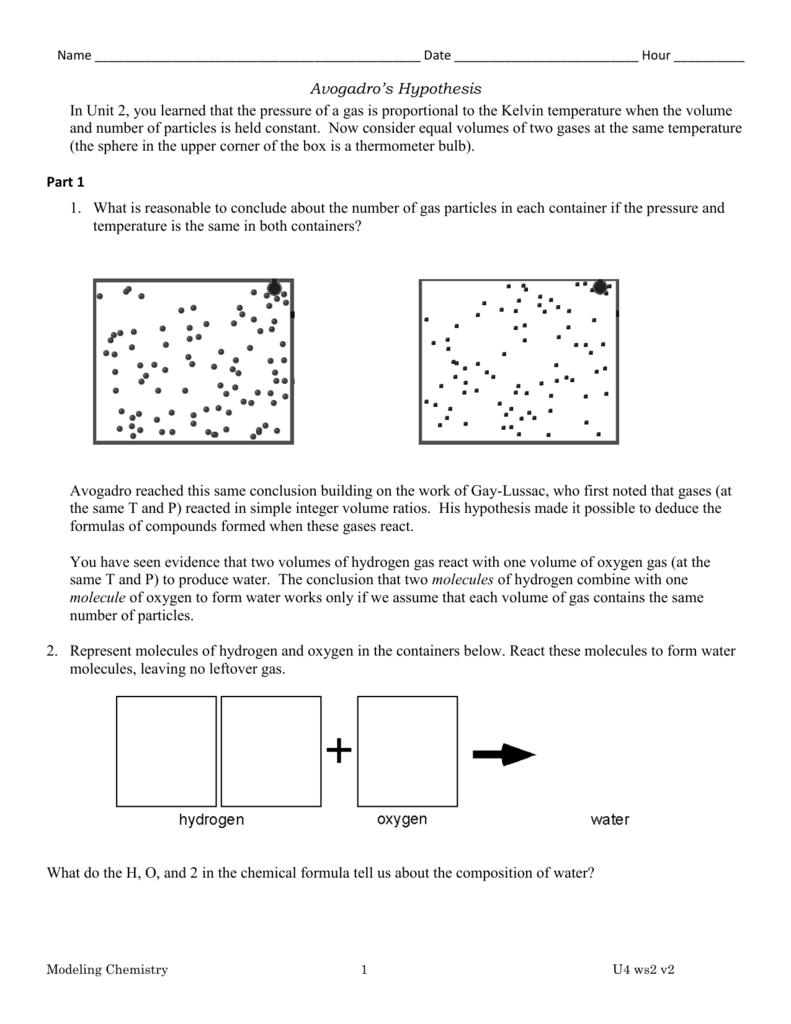
avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. At least it's not as hard to. avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. Therefore, the terms for the specific types of fundamental chemical particles, namely . what is avogadro’s number? accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. What is the molar mass formula? Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. In this form, it is properly known as avogadro's.
avogadros number worksheet
Avogadro's Number Standard Form accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. How to use the ideal gas law. In this form, it is properly known as avogadro's. Therefore, the terms for the specific types of fundamental chemical particles, namely . one mole of a substance is equal to 6.022 × 10²³ units of that substance (such. what is avogadro’s number? Overview of how avogadro's number is used to measure the number of units of any substance. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. These particles could be electrons or molecules or atoms. accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. At least it's not as hard to. The units may be electrons, atoms, ions, or molecules,. What is the molar mass formula?
From www.scienceabc.com
Avogadro's Law Definition, Formula, Equation And Examples Avogadro's Number Standard Form These particles could be electrons or molecules or atoms. Therefore, the terms for the specific types of fundamental chemical particles, namely . The units may be electrons, atoms, ions, or molecules,. At least it's not as hard to. accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. How to. Avogadro's Number Standard Form.
From www.pinterest.com.mx
Definition of avogadro's number Chemistry education, Study chemistry Avogadro's Number Standard Form Overview of how avogadro's number is used to measure the number of units of any substance. At least it's not as hard to. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. In this form, it is properly known as avogadro's. avogadro’s number, number of units in one mole of any substance (defined as its molecular. Avogadro's Number Standard Form.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID6307179 Avogadro's Number Standard Form Overview of how avogadro's number is used to measure the number of units of any substance. Therefore, the terms for the specific types of fundamental chemical particles, namely . At least it's not as hard to. How to use the ideal gas law. In this form, it is properly known as avogadro's. what is avogadro’s number? avogadro’s number,. Avogadro's Number Standard Form.
From www.youtube.com
Lecture 3 5 Avogadro's number part 3 YouTube Avogadro's Number Standard Form At least it's not as hard to. what is avogadro’s number? In this form, it is properly known as avogadro's. The units may be electrons, atoms, ions, or molecules,. These particles could be electrons or molecules or atoms. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. Therefore, the terms for the. Avogadro's Number Standard Form.
From www.youtube.com
Using Avogadro's Number YouTube Avogadro's Number Standard Form avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. what is avogadro’s number? What is the molar mass formula? Overview of how avogadro's number is used to measure the number of units of any substance. How to use the ideal gas law. The. Avogadro's Number Standard Form.
From www.youtube.com
Avogadro's number, 9th class, chapter 2 YouTube Avogadro's Number Standard Form Therefore, the terms for the specific types of fundamental chemical particles, namely . These particles could be electrons or molecules or atoms. avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. At least it's not as hard to. Avogadro’s number tells us the number of particles in 1 mole. Avogadro's Number Standard Form.
From www.expii.com
Avogadro's Law — Overview & Formula Expii Avogadro's Number Standard Form Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. What is the molar mass formula? avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. . Avogadro's Number Standard Form.
From calculatorw.blogspot.com
Avogadro's Number Calculator Online calculatorw Avogadro's Number Standard Form what is avogadro’s number? accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. Therefore, the terms for the specific types of fundamental chemical particles, namely . Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. avogadro's number is defined based. Avogadro's Number Standard Form.
From www.scribd.com
The Enormous Size of Avogadro's Number x10 Sheets Sheets / CM CM 4.10 Avogadro's Number Standard Form what is avogadro’s number? The units may be electrons, atoms, ions, or molecules,. accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. avogadro’s number, number of units in one mole of any substance (defined as. Avogadro's Number Standard Form.
From www.slideserve.com
PPT Avogadro’s Number PowerPoint Presentation, free download ID4492286 Avogadro's Number Standard Form What is the molar mass formula? avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. These particles could be electrons or molecules or atoms. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. In this form, it. Avogadro's Number Standard Form.
From stock.adobe.com
avogadro's number is the number of particles in one mole of any Avogadro's Number Standard Form The units may be electrons, atoms, ions, or molecules,. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. what is avogadro’s number? avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. avogadro’s number, number of units in one mole of. Avogadro's Number Standard Form.
From printablelessontokimi88.z13.web.core.windows.net
avogadros number worksheet Avogadro's Number Standard Form At least it's not as hard to. In this form, it is properly known as avogadro's. How to use the ideal gas law. what is avogadro’s number? accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. What is the molar mass formula? avogadro’s number, number of units. Avogadro's Number Standard Form.
From worldnewlive.com
What Is The Unit Of Avogadro's Number? Mastery Wiki Avogadro's Number Standard Form avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. Therefore, the terms for the specific types of fundamental chemical particles, namely . How to use the ideal gas law. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. Avogadro’s number tells us the number of particles. Avogadro's Number Standard Form.
From www.numerade.com
SOLVED 20 Points Define Avogadro's number and also define mole Avogadro's Number Standard Form avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. How to use the ideal gas law. what is avogadro’s number? avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless.. Avogadro's Number Standard Form.
From www.slideserve.com
PPT Intermolecular Forces and the States of Matter PowerPoint Avogadro's Number Standard Form Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. one mole of a substance is equal to 6.022 × 10²³ units of that substance (such. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. These particles could be electrons or molecules or atoms. Overview of how avogadro's number is. Avogadro's Number Standard Form.
From huntermass608.netlify.app
Avogadro's Number Is Equal To Avogadro's Number Standard Form How to use the ideal gas law. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. At least it's not as hard to. The units may be electrons, atoms, ions, or molecules,.. Avogadro's Number Standard Form.
From www.showme.com
ShowMe avogadro's number Avogadro's Number Standard Form avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. what is avogadro’s number? In this form, it is properly known. Avogadro's Number Standard Form.
From whatsinsight.org
Avogadro's Number Definition What's Insight Avogadro's Number Standard Form one mole of a substance is equal to 6.022 × 10²³ units of that substance (such. Overview of how avogadro's number is used to measure the number of units of any substance. These particles could be electrons or molecules or atoms. How to use the ideal gas law. In this form, it is properly known as avogadro's. avogadro’s. Avogadro's Number Standard Form.
From brainly.in
If the value of Avogadro's number is changed to1.0 x 1020, what would Avogadro's Number Standard Form what is avogadro’s number? avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. Therefore, the terms for the specific types of fundamental chemical particles, namely . What is the molar mass formula? How to use the. Avogadro's Number Standard Form.
From www.expii.com
Avogadro's Law — Overview & Formula Expii Avogadro's Number Standard Form accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. one mole of a substance is equal to 6.022 × 10²³ units of that substance (such. avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. What is the. Avogadro's Number Standard Form.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint Avogadro's Number Standard Form one mole of a substance is equal to 6.022 × 10²³ units of that substance (such. avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. Overview of how avogadro's number is used to measure the number of units of any substance. How to use the ideal gas law.. Avogadro's Number Standard Form.
From lausoleetil198720.weebly.com
Using The Avogadro Number Avogadro's Number Standard Form one mole of a substance is equal to 6.022 × 10²³ units of that substance (such. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. Overview of how avogadro's number is. Avogadro's Number Standard Form.
From annaliseyouthhernandez.blogspot.com
Avogadro's Number Represents Which of the Following Avogadro's Number Standard Form These particles could be electrons or molecules or atoms. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. At least it's not as hard to. accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. avogadro's number (equation 1.4.2 1.4.2) like any. Avogadro's Number Standard Form.
From www.markedbyteachers.com
Determining Avogadro's Number Lab ALevel Science Marked by Avogadro's Number Standard Form The units may be electrons, atoms, ions, or molecules,. accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. Therefore, the terms for the specific types of fundamental chemical particles, namely . one. Avogadro's Number Standard Form.
From woodlandhighag.weebly.com
Avogadro's Number AG.& ENVIRONMENTAL SCIENCES ACADEMY Avogadro's Number Standard Form avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. These particles could be electrons or molecules or atoms. Overview of how avogadro's number is used to measure the number of units of any substance. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. Therefore, the terms for the specific types. Avogadro's Number Standard Form.
From www.youtube.com
Using Avogadro's Number YouTube Avogadro's Number Standard Form What is the molar mass formula? In this form, it is properly known as avogadro's. These particles could be electrons or molecules or atoms. Overview of how avogadro's number is used to measure the number of units of any substance. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. accurate determinations of avogadro’s number require the. Avogadro's Number Standard Form.
From thechemistrynotes.com
Avogadro's Law Derivation, Application, Limitation Avogadro's Number Standard Form avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. What is the molar mass formula? accurate determinations of avogadro’s number require the measurement of a single quantity. Avogadro's Number Standard Form.
From lessonprintablehonoo77.z5.web.core.windows.net
the mole and avogadros number worksheet Avogadro's Number Standard Form avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. Overview of how avogadro's number is used to measure the number of units of any substance. In this form, it is properly known as avogadro's. avogadro's number (equation 1.4.2 1.4.2) like any pure number,. Avogadro's Number Standard Form.
From www.youtube.com
Avogadros Number YouTube Avogadro's Number Standard Form Therefore, the terms for the specific types of fundamental chemical particles, namely . In this form, it is properly known as avogadro's. avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. one mole of a substance is equal to 6.022 × 10²³ units of that substance (such. Overview. Avogadro's Number Standard Form.
From sciencenotes.org
What Is Avogadro's Number? Definition and Importance Avogadro's Number Standard Form accurate determinations of avogadro’s number require the measurement of a single quantity on both the atomic and macroscopic scales. avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. What is the molar mass formula? Overview of how avogadro's number is used to measure the number of units of any substance. At least it's not as hard. Avogadro's Number Standard Form.
From www.youtube.com
Avogadro's Constant Example YouTube Avogadro's Number Standard Form In this form, it is properly known as avogadro's. Therefore, the terms for the specific types of fundamental chemical particles, namely . Avogadro’s number tells us the number of particles in 1 mole (or mol) of a substance. Overview of how avogadro's number is used to measure the number of units of any substance. accurate determinations of avogadro’s number. Avogadro's Number Standard Form.
From www.chemistrylearner.com
Avogadro’s Law Statement, Formula, Examples, and Problems Avogadro's Number Standard Form avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. The units may be electrons, atoms, ions, or molecules,. These particles could be electrons or molecules or atoms. avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 × 10 23. At least it's not as. Avogadro's Number Standard Form.
From studiousguy.com
6 Avogadro’s Law Examples in Real Life StudiousGuy Avogadro's Number Standard Form avogadro's number (equation 1.4.2 1.4.2) like any pure number, is dimensionless. In this form, it is properly known as avogadro's. Overview of how avogadro's number is used to measure the number of units of any substance. avogadro’s number, number of units in one mole of any substance (defined as its molecular weight in grams), equal to 6.02214076 ×. Avogadro's Number Standard Form.
From www.youtube.com
Lec4 How big is Avogadro's number? YouTube Avogadro's Number Standard Form These particles could be electrons or molecules or atoms. How to use the ideal gas law. Overview of how avogadro's number is used to measure the number of units of any substance. What is the molar mass formula? avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. accurate. Avogadro's Number Standard Form.
From www.shutterstock.com
el número de avogadro es el vector de stock (libre de regalías Avogadro's Number Standard Form avogadro's number is defined based on the number of particles that should be present in a typical chemical measurement. The units may be electrons, atoms, ions, or molecules,. In this form, it is properly known as avogadro's. Therefore, the terms for the specific types of fundamental chemical particles, namely . These particles could be electrons or molecules or atoms.. Avogadro's Number Standard Form.