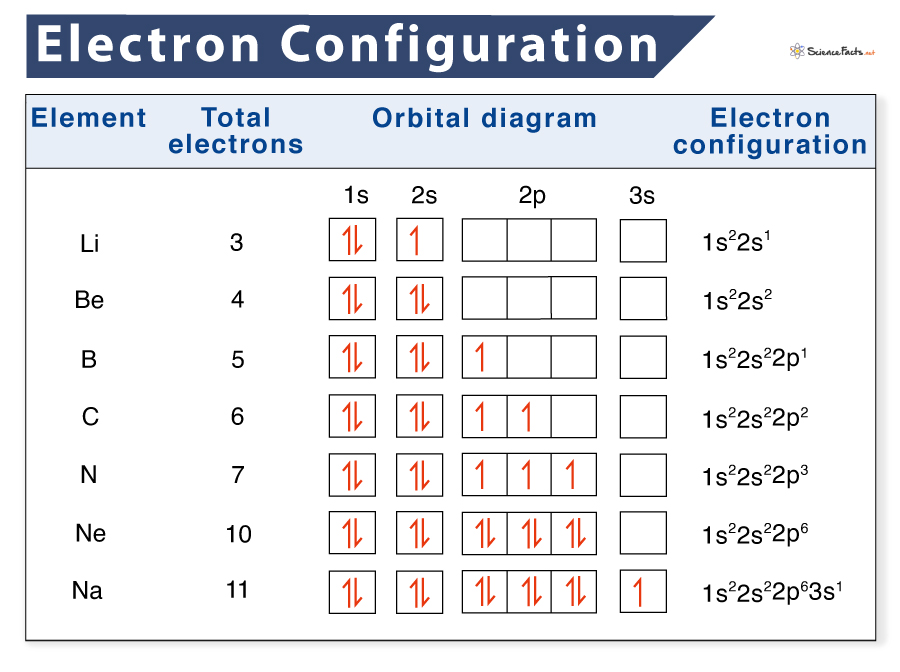
Iron Electron Configuration Chart . Since 1s can only hold two. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Nitrates and iron chloride are used as industrial reagents. This page shows the electron configurations of the neutral gaseous atoms in their ground. the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. The iron sulfate is used in the fungicide. the electron configuration for any chemical element is basically the process that defines the electrons. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe.
from www.sciencefacts.net
in writing the electron configuration for iron the first two electrons will go in the 1s orbital. This page shows the electron configurations of the neutral gaseous atoms in their ground. the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The iron sulfate is used in the fungicide. Nitrates and iron chloride are used as industrial reagents. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. the electron configuration for any chemical element is basically the process that defines the electrons. Since 1s can only hold two. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe.
Electron Configuration Definition, Examples, Chart, and Diagram
Iron Electron Configuration Chart The iron sulfate is used in the fungicide. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; The iron sulfate is used in the fungicide. the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Nitrates and iron chloride are used as industrial reagents. the electron configuration for any chemical element is basically the process that defines the electrons. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Since 1s can only hold two. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. This page shows the electron configurations of the neutral gaseous atoms in their ground.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry Iron Electron Configuration Chart This page shows the electron configurations of the neutral gaseous atoms in their ground. Since 1s can only hold two. Nitrates and iron chloride are used as industrial reagents. the electron configuration for any chemical element is basically the process that defines the electrons. The iron sulfate is used in the fungicide. Fe2+ contains 2 fewer electrons compared to. Iron Electron Configuration Chart.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and Iron Electron Configuration Chart This page shows the electron configurations of the neutral gaseous atoms in their ground. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Nitrates and iron chloride are used as industrial reagents. in writing. Iron Electron Configuration Chart.
From wou.edu
CH104 Chapter 2 Atoms and The Periodic Table Chemistry Iron Electron Configuration Chart the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Nitrates. Iron Electron Configuration Chart.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Iron Electron Configuration Chart The iron sulfate is used in the fungicide. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; the electron configuration for any chemical element is basically the process that defines the electrons. the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. in. Iron Electron Configuration Chart.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic Iron Electron Configuration Chart the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. The iron sulfate is used in the fungicide. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. the arrangement of electrons in the orbitals of an atom is called the electron configuration of the. Iron Electron Configuration Chart.
From socratic.org
How do you calculate valence electrons for transition metals? Socratic Iron Electron Configuration Chart Since 1s can only hold two. The iron sulfate is used in the fungicide. the electron configuration for any chemical element is basically the process that defines the electrons. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. in writing the electron configuration. Iron Electron Configuration Chart.
From sciencenotes.org
List of Electron Configurations of Elements Iron Electron Configuration Chart in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The iron sulfate is used in the fungicide. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; Nitrates and iron chloride are used as industrial reagents. . Iron Electron Configuration Chart.
From ar.inspiredpencil.com
Electron Configuration Of Iron Iron Electron Configuration Chart the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Nitrates and iron chloride are used as industrial reagents. Since 1s can only hold two. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. in writing the electron configuration for iron the first two. Iron Electron Configuration Chart.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Iron Electron Configuration Chart the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. in writing the electron configuration for. Iron Electron Configuration Chart.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry Iron Electron Configuration Chart the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Nitrates and iron chloride are used as industrial reagents. This page shows the electron configurations of the neutral gaseous atoms in their ground. Since 1s can only hold two. the electron configuration for any chemical element is. Iron Electron Configuration Chart.
From socratic.org
How do you find electron configuration using the periodic table? Socratic Iron Electron Configuration Chart Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. The iron sulfate is used in the fungicide. This page shows the electron configurations of the neutral gaseous atoms in their ground. Nitrates and iron chloride are used as industrial reagents. in writing the electron configuration for iron the first two electrons will go in the 1s. Iron Electron Configuration Chart.
From sciencenotes.org
List of Electron Configurations of Elements Iron Electron Configuration Chart The iron sulfate is used in the fungicide. Nitrates and iron chloride are used as industrial reagents. the electron configuration for any chemical element is basically the process that defines the electrons. Since 1s can only hold two. the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6. Iron Electron Configuration Chart.
From www.periodictableprintable.com
Periodic Table With Electron Structure Of Energy Levels 2023 Periodic Iron Electron Configuration Chart the electron configuration for any chemical element is basically the process that defines the electrons. Since 1s can only hold two. This page shows the electron configurations of the neutral gaseous atoms in their ground. The iron sulfate is used in the fungicide. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. the arrangement of. Iron Electron Configuration Chart.
From alisaoimeyers.blogspot.com
Electronic Configuration of Elements AlisaoiMeyers Iron Electron Configuration Chart The iron sulfate is used in the fungicide. This page shows the electron configurations of the neutral gaseous atoms in their ground. the electron configuration for any chemical element is basically the process that defines the electrons. the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5.. Iron Electron Configuration Chart.
From socratic.org
Question 9267e Socratic Iron Electron Configuration Chart Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; in writing the electron configuration for iron the first two electrons will go in the 1s orbital. the electron configuration for any chemical element is basically the process that defines the electrons. the. Iron Electron Configuration Chart.
From sciencenotes.org
Periodic Table with Electron Configurations 2015 Iron Electron Configuration Chart 119 rows shorthand electron configuration full electron configuration electron shell arrangement; the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. Since 1s can only hold two. The iron sulfate is used. Iron Electron Configuration Chart.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Iron Electron Configuration Chart the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. This page shows the electron configurations of the neutral gaseous atoms in their ground. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. The iron sulfate is used in. Iron Electron Configuration Chart.
From scientifictutor.org
Chem Electron Configuration Diagrams Scientific Tutor Iron Electron Configuration Chart the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. the electron configuration for any chemical element is basically the process that defines the electrons. The iron sulfate is used in the fungicide. 119 rows shorthand electron configuration full electron configuration electron shell arrangement; Since 1s can only hold. Iron Electron Configuration Chart.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts Iron Electron Configuration Chart This page shows the electron configurations of the neutral gaseous atoms in their ground. Nitrates and iron chloride are used as industrial reagents. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. Since 1s can only hold two. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. . Iron Electron Configuration Chart.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts Iron Electron Configuration Chart The iron sulfate is used in the fungicide. Fe2+ contains 2 fewer electrons compared to the electronic configuration of fe. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. This page shows the electron configurations of the neutral gaseous atoms in their ground. Nitrates and iron chloride are used as industrial. Iron Electron Configuration Chart.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Electron Configuration Chart The iron sulfate is used in the fungicide. the electron configuration for any chemical element is basically the process that defines the electrons. in writing the electron configuration for iron the first two electrons will go in the 1s orbital. This page shows the electron configurations of the neutral gaseous atoms in their ground. 119 rows shorthand. Iron Electron Configuration Chart.
From byjus.com
Electronic Configuration How To Write Electron ConfigurationChemistry Iron Electron Configuration Chart The iron sulfate is used in the fungicide. the electronic configuration of fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6 and fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5. Since 1s can only hold two. the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. 119 rows shorthand electron. Iron Electron Configuration Chart.