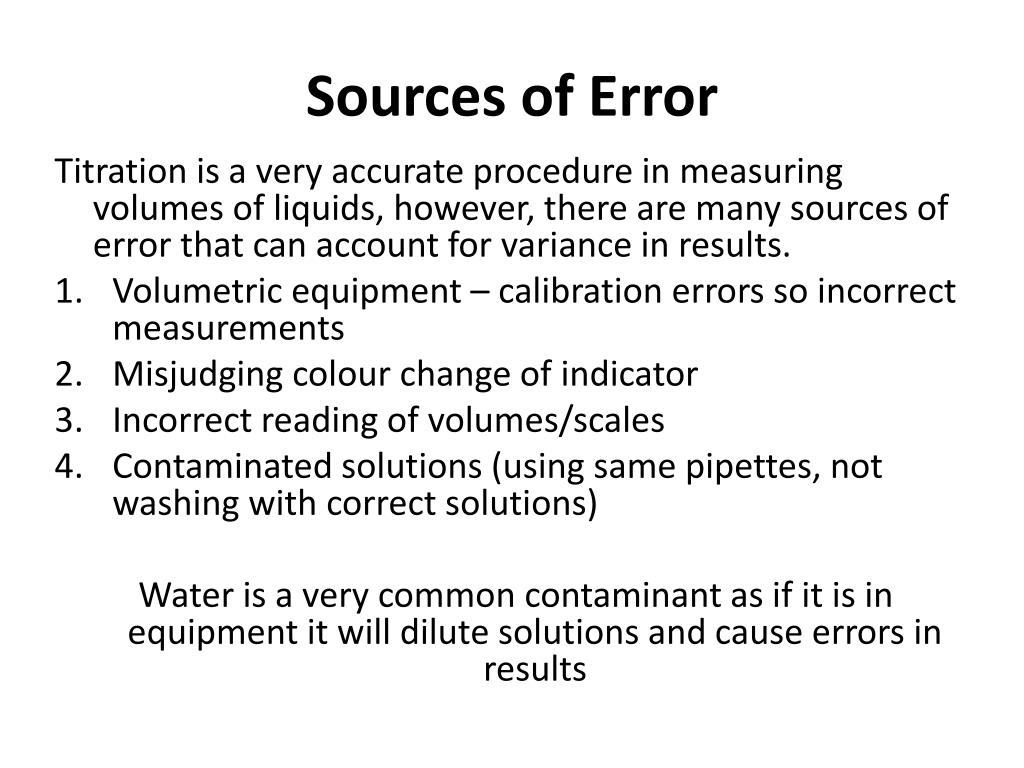
Titration Error Formula . propagate uncertainty for common mathematical operations including: 9.4k views 3 years ago titration lab experiment. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: An error is the difference between a value or quantity obtained in an experiment and an. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. These can be adjusted for by. mettler toledo titration guide 7 avoiding titration errors 3. Percent error is determined by taking the absolute value of. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. Avoiding titration errors many errors in analytical analysis arise. then, there are errors that can be connected with volumetric glass accuracy.
from www.slideserve.com
Avoiding titration errors many errors in analytical analysis arise. mettler toledo titration guide 7 avoiding titration errors 3. 9.4k views 3 years ago titration lab experiment. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. suppose that a titration is performed and \(20.70 \: Percent error is determined by taking the absolute value of. An error is the difference between a value or quantity obtained in an experiment and an. propagate uncertainty for common mathematical operations including: \ce{naoh}\) is required to reach the end. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound.
PPT Volumetric Analysis (Titration) PowerPoint Presentation, free
Titration Error Formula suppose that a titration is performed and \(20.70 \: 9.4k views 3 years ago titration lab experiment. suppose that a titration is performed and \(20.70 \: then, there are errors that can be connected with volumetric glass accuracy. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. mettler toledo titration guide 7 avoiding titration errors 3. Avoiding titration errors many errors in analytical analysis arise. Percent error is determined by taking the absolute value of. These can be adjusted for by. propagate uncertainty for common mathematical operations including: An error is the difference between a value or quantity obtained in an experiment and an. \ce{naoh}\) is required to reach the end.
From www.easybiologyclass.com
What is Titration Curve? What is pKa? EasyBiologyClass Titration Error Formula Avoiding titration errors many errors in analytical analysis arise. These can be adjusted for by. propagate uncertainty for common mathematical operations including: \ce{naoh}\) is required to reach the end. mettler toledo titration guide 7 avoiding titration errors 3. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. titrations are. Titration Error Formula.
From www.chegg.com
Solved Review the list of common titration errors. Determine Titration Error Formula \ce{naoh}\) is required to reach the end. 9.4k views 3 years ago titration lab experiment. propagate uncertainty for common mathematical operations including: Percent error is determined by taking the absolute value of. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. An error is the difference between a value or quantity. Titration Error Formula.
From www.chegg.com
Solved Fit Page Height 8* Sources of Error in Titrations Titration Error Formula titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. then, there are errors that can be connected with volumetric glass accuracy. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. Percent error is determined by taking the absolute value of. . Titration Error Formula.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Error Formula These can be adjusted for by. propagate uncertainty for common mathematical operations including: Percent error is determined by taking the absolute value of. 9.4k views 3 years ago titration lab experiment. \ce{naoh}\) is required to reach the end. suppose that a titration is performed and \(20.70 \: the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh}. Titration Error Formula.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Error Formula Percent error is determined by taking the absolute value of. propagate uncertainty for common mathematical operations including: the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. Avoiding titration errors many errors in analytical analysis arise. mettler toledo titration guide 7 avoiding titration errors 3. titrations are carried out to. Titration Error Formula.
From www.youtube.com
Normality Factor 08 Titration Class 11th & 12th YouTube Titration Error Formula the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. Avoiding titration errors many errors in analytical analysis arise. propagate uncertainty for common mathematical operations including: suppose that a titration is performed and \(20.70 \: These can be adjusted for by. mettler toledo titration guide 7 avoiding titration errors 3.. Titration Error Formula.
From www.slideserve.com
PPT Volumetric Analysis (Titration) PowerPoint Presentation, free Titration Error Formula These can be adjusted for by. Avoiding titration errors many errors in analytical analysis arise. propagate uncertainty for common mathematical operations including: suppose that a titration is performed and \(20.70 \: 9.4k views 3 years ago titration lab experiment. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. \ce{naoh}\) is. Titration Error Formula.
From chem.libretexts.org
Complexation Titration Chemistry LibreTexts Titration Error Formula Percent error is determined by taking the absolute value of. 9.4k views 3 years ago titration lab experiment. \ce{naoh}\) is required to reach the end. then, there are errors that can be connected with volumetric glass accuracy. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. Avoiding titration errors many errors. Titration Error Formula.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 Titration Error Formula Percent error is determined by taking the absolute value of. then, there are errors that can be connected with volumetric glass accuracy. \ce{naoh}\) is required to reach the end. mettler toledo titration guide 7 avoiding titration errors 3. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. An error is. Titration Error Formula.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Titration Error Formula \ce{naoh}\) is required to reach the end. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. Percent error is determined by taking the absolute value of. Avoiding titration errors many errors in analytical analysis arise. suppose that a titration is performed and \(20.70 \: the equation is \[. Titration Error Formula.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Error Formula Avoiding titration errors many errors in analytical analysis arise. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. These can be adjusted for by. suppose that a titration is performed and \(20.70 \: the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6}. Titration Error Formula.
From studylib.net
AcidBase Titration Answers, Sources of Error Titration Error Formula An error is the difference between a value or quantity obtained in an experiment and an. 9.4k views 3 years ago titration lab experiment. Avoiding titration errors many errors in analytical analysis arise. propagate uncertainty for common mathematical operations including: These can be adjusted for by. suppose that a titration is performed and \(20.70 \: the equation. Titration Error Formula.
From labthink.netlify.app
What is titration and how does it work Titration Error Formula An error is the difference between a value or quantity obtained in an experiment and an. mettler toledo titration guide 7 avoiding titration errors 3. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na. Titration Error Formula.
From itnewstoday.net
Fixed How To Fix Experimental Error Sources In Titration. IT News Today Titration Error Formula titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. Percent error is determined by taking the absolute value of. 9.4k views 3 years ago titration lab experiment. propagate uncertainty for common mathematical operations including: mettler toledo titration guide 7 avoiding titration errors 3. then, there are errors. Titration Error Formula.
From chem.libretexts.org
Quantitative Analysis Nuts and Bolts Chemistry LibreTexts Titration Error Formula \ce{naoh}\) is required to reach the end. Avoiding titration errors many errors in analytical analysis arise. 9.4k views 3 years ago titration lab experiment. mettler toledo titration guide 7 avoiding titration errors 3. suppose that a titration is performed and \(20.70 \: These can be adjusted for by. An error is the difference between a value or quantity. Titration Error Formula.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Error Formula 9.4k views 3 years ago titration lab experiment. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. then, there are errors that can be connected with volumetric glass accuracy. Avoiding titration errors many errors in analytical analysis arise. titrations are carried out to determine the concentration, \text {m}_ {\text {r}},. Titration Error Formula.
From chem.libretexts.org
3.7 AcidBase Titrations Chemistry LibreTexts Titration Error Formula mettler toledo titration guide 7 avoiding titration errors 3. An error is the difference between a value or quantity obtained in an experiment and an. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. \ce{naoh}\) is required to reach the end. propagate uncertainty for common mathematical operations including: Percent error. Titration Error Formula.
From www.youtube.com
Errors in titrations YouTube Titration Error Formula These can be adjusted for by. Percent error is determined by taking the absolute value of. mettler toledo titration guide 7 avoiding titration errors 3. An error is the difference between a value or quantity obtained in an experiment and an. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown. Titration Error Formula.
From www.studocu.com
Error Analysis Example in Titration Chem 3300 Studocu Titration Error Formula titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. An error is the difference between a value or quantity obtained in an experiment and an. Avoiding titration errors many errors in analytical analysis. Titration Error Formula.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Titration Error Formula Avoiding titration errors many errors in analytical analysis arise. propagate uncertainty for common mathematical operations including: then, there are errors that can be connected with volumetric glass accuracy. An error is the difference between a value or quantity obtained in an experiment and an. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{. Titration Error Formula.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Error Formula suppose that a titration is performed and \(20.70 \: These can be adjusted for by. 9.4k views 3 years ago titration lab experiment. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. \ce{naoh}\) is required to reach the end. then, there are errors that can be connected with. Titration Error Formula.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Error Formula \ce{naoh}\) is required to reach the end. 9.4k views 3 years ago titration lab experiment. An error is the difference between a value or quantity obtained in an experiment and an. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. suppose that a titration is performed and \(20.70 \:. Titration Error Formula.
From www.nagwa.com
Lesson Titration Calculations Nagwa Titration Error Formula the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. mettler toledo titration guide 7 avoiding titration errors 3. propagate uncertainty for common mathematical operations including: These can be adjusted for by. 9.4k views 3 years ago titration lab experiment. suppose that a titration is performed and \(20.70 \: . Titration Error Formula.
From www.youtube.com
Year 12 Chemistry Revision Titrating Using the Burette to make an Titration Error Formula suppose that a titration is performed and \(20.70 \: then, there are errors that can be connected with volumetric glass accuracy. propagate uncertainty for common mathematical operations including: These can be adjusted for by. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. \ce{naoh}\) is required to. Titration Error Formula.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Error Formula titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. \ce{naoh}\) is required to reach the end. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. An error is the difference between a value or quantity obtained in an experiment and an. Percent. Titration Error Formula.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Error Formula An error is the difference between a value or quantity obtained in an experiment and an. mettler toledo titration guide 7 avoiding titration errors 3. then, there are errors that can be connected with volumetric glass accuracy. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. Avoiding titration. Titration Error Formula.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Error Formula titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. then, there are errors that can be connected with volumetric glass accuracy. suppose that a titration is performed and \(20.70 \: propagate uncertainty for common mathematical operations including: 9.4k views 3 years ago titration lab experiment. These can. Titration Error Formula.
From www.tes.com
Measurement calculations/uncertainty for Titrations AS Chemistry Titration Error Formula 9.4k views 3 years ago titration lab experiment. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. then, there are errors that can be connected with volumetric glass accuracy. An error is the difference between a value or quantity obtained in an experiment and an. \ce{naoh}\) is required to. Titration Error Formula.
From www.showme.com
Percent error part 2 Chemistry, Science, Stoichiometry ShowMe Titration Error Formula suppose that a titration is performed and \(20.70 \: An error is the difference between a value or quantity obtained in an experiment and an. 9.4k views 3 years ago titration lab experiment. mettler toledo titration guide 7 avoiding titration errors 3. propagate uncertainty for common mathematical operations including: Avoiding titration errors many errors in analytical analysis. Titration Error Formula.
From www.scielo.br
SciELO Brasil Potentiometric Titration and OutOfEquilibrium pH Titration Error Formula \ce{naoh}\) is required to reach the end. Percent error is determined by taking the absolute value of. suppose that a titration is performed and \(20.70 \: then, there are errors that can be connected with volumetric glass accuracy. 9.4k views 3 years ago titration lab experiment. titrations are carried out to determine the concentration, \text {m}_ {\text. Titration Error Formula.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Titration Error Formula Avoiding titration errors many errors in analytical analysis arise. suppose that a titration is performed and \(20.70 \: titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. These can be adjusted for by. propagate uncertainty for common mathematical operations including: An error is the difference between a value. Titration Error Formula.
From studylib.net
CDCP 02.28.11 Titration Excel Practice Example Titration Error Formula \ce{naoh}\) is required to reach the end. the equation is \[ \text{c}_{6} \text{h}_{8} \text{o}_{6} (aq) + \text{naoh} (aq) \rightarrow \text{ na c}_{6} \text{h}_{7}. propagate uncertainty for common mathematical operations including: mettler toledo titration guide 7 avoiding titration errors 3. An error is the difference between a value or quantity obtained in an experiment and an. suppose. Titration Error Formula.
From www.slideserve.com
PPT Percent Error PowerPoint Presentation, free download ID5581807 Titration Error Formula An error is the difference between a value or quantity obtained in an experiment and an. suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. then, there are errors that can be. Titration Error Formula.
From www.youtube.com
Titration Calculations YouTube Titration Error Formula 9.4k views 3 years ago titration lab experiment. Avoiding titration errors many errors in analytical analysis arise. titrations are carried out to determine the concentration, \text {m}_ {\text {r}}, or formula of an unknown compound. These can be adjusted for by. mettler toledo titration guide 7 avoiding titration errors 3. Percent error is determined by taking the absolute. Titration Error Formula.
From www.youtube.com
Random and Systematic Errors in Titrations YouTube Titration Error Formula 9.4k views 3 years ago titration lab experiment. suppose that a titration is performed and \(20.70 \: An error is the difference between a value or quantity obtained in an experiment and an. These can be adjusted for by. \ce{naoh}\) is required to reach the end. mettler toledo titration guide 7 avoiding titration errors 3. propagate uncertainty. Titration Error Formula.