Standard Enthalpy Of Formation Gallium . The formation of any chemical can be as a reaction from the corresponding elements: Mallard, eds, nist chemistry webbook, nist standard reference database. All standard state, 25 °c and 1 bar (written to 1 decimal place). Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79.
from chem.libretexts.org
Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. All standard state, 25 °c and 1 bar (written to 1 decimal place). The formation of any chemical can be as a reaction from the corresponding elements: Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions.
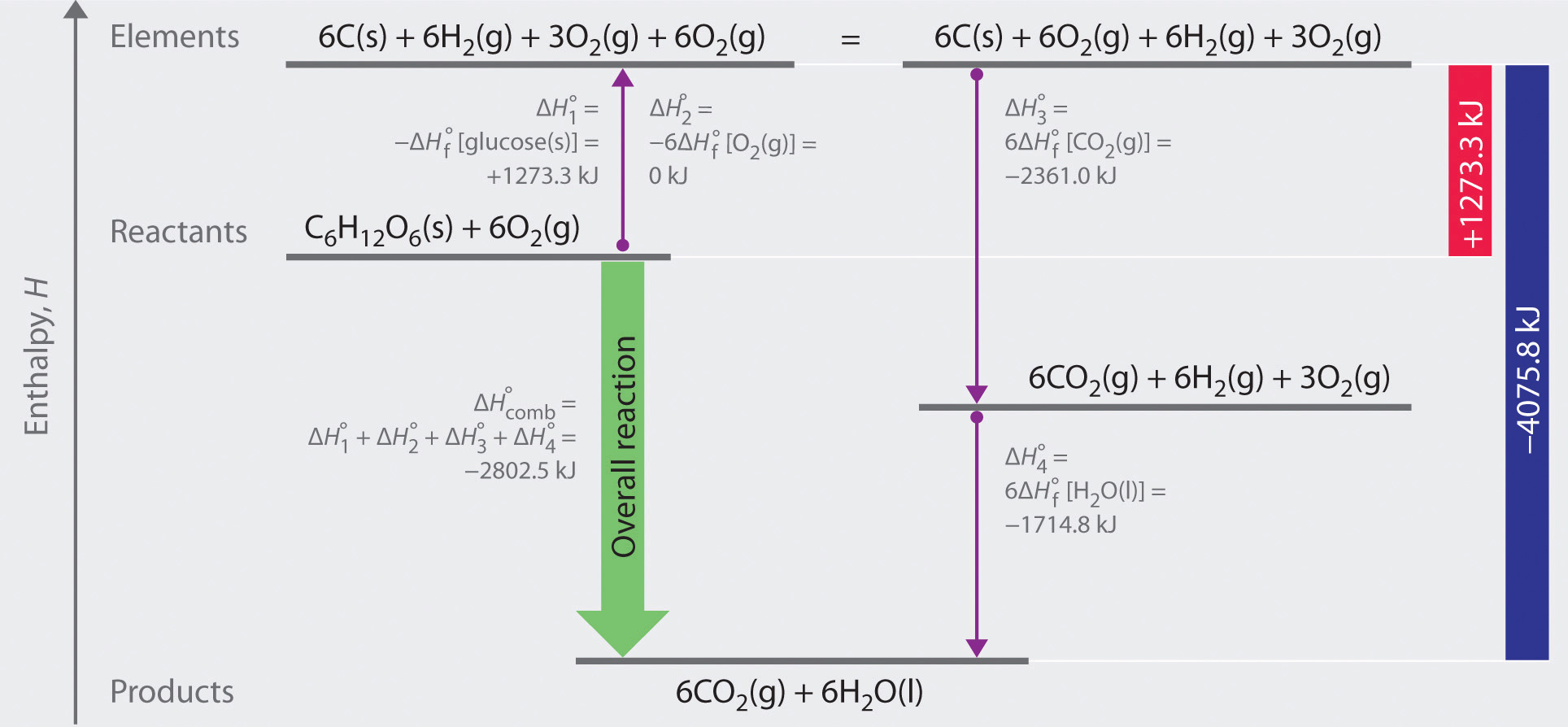
5.7 Enthalpies of Formation Chemistry LibreTexts
Standard Enthalpy Of Formation Gallium The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The formation of any chemical can be as a reaction from the corresponding elements: The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard, eds, nist chemistry webbook, nist standard reference database. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Gallium 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The formation of any. Standard Enthalpy Of Formation Gallium.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Gallium 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation, also known as the heat of formation, is the. Standard Enthalpy Of Formation Gallium.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Gallium Mallard, eds, nist chemistry webbook, nist standard reference database. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25. Standard Enthalpy Of Formation Gallium.
From narodnatribuna.info
Enthalpy Equation Standard Enthalpy Of Formation Gallium The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy. Standard Enthalpy Of Formation Gallium.
From www.w3schools.blog
Standard enthalpy of ionization W3schools Standard Enthalpy Of Formation Gallium Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. All standard state, 25 °c and 1 bar (written to 1 decimal place). Enthalpy of formation (δhf) is. Standard Enthalpy Of Formation Gallium.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Gallium The formation of any chemical can be as a reaction from the corresponding elements: Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol. Standard Enthalpy Of Formation Gallium.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Gallium Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standand enthalpies of formation &. Standard Enthalpy Of Formation Gallium.
From www.semanticscholar.org
Figure 2 from Enthalpy of Formation of GalliumGermaniumTin Liquid Standard Enthalpy Of Formation Gallium Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements. Standard Enthalpy Of Formation Gallium.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Gallium Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The formation of any chemical can be as a reaction from the corresponding elements: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Gallium.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Gallium Mallard, eds, nist chemistry webbook, nist standard reference database. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. Enthalpy of formation (δhf). Standard Enthalpy Of Formation Gallium.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Gallium The formation of any chemical can be as a reaction from the corresponding elements: 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq. Standard Enthalpy Of Formation Gallium.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Gallium The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79. The standard enthalpy. Standard Enthalpy Of Formation Gallium.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Gallium Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Enthalpy Of Formation Gallium.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Gallium The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The formation of any chemical. Standard Enthalpy Of Formation Gallium.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Gallium The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Mallard, eds,. Standard Enthalpy Of Formation Gallium.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Gallium Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Mallard, eds, nist chemistry webbook, nist standard reference database. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is. Standard Enthalpy Of Formation Gallium.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Standard Enthalpy Of Formation Gallium All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Standard Enthalpy Of Formation Gallium.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Gallium The formation of any chemical can be as a reaction from the corresponding elements: The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation is a measure of the energy released or. Standard Enthalpy Of Formation Gallium.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Gallium Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. Mallard, eds, nist chemistry webbook, nist standard reference database. All standard. Standard Enthalpy Of Formation Gallium.
From www.slideserve.com
PPT STATE FUNCTIONS and ENTHALPY PowerPoint Presentation, free Standard Enthalpy Of Formation Gallium Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Mallard, eds, nist chemistry webbook, nist standard reference database. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of. Standard Enthalpy Of Formation Gallium.
From www.semanticscholar.org
Figure 1 from Enthalpy of Formation of GalliumGermaniumTin Liquid Standard Enthalpy Of Formation Gallium Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the.. Standard Enthalpy Of Formation Gallium.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Gallium 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying. Standard Enthalpy Of Formation Gallium.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation Gallium Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation. Standard Enthalpy Of Formation Gallium.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Gallium The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The formation of any. Standard Enthalpy Of Formation Gallium.
From cewuaeqb.blob.core.windows.net
How Do You Work Out Standard Enthalpy Of Formation at Connie Stroud blog Standard Enthalpy Of Formation Gallium The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms. Standard Enthalpy Of Formation Gallium.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Gallium The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. All standard state, 25 °c and 1 bar (written to 1 decimal place). Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such. Standard Enthalpy Of Formation Gallium.
From www.youtube.com
6.6 Standard Enthalpy of Formation and Reaction YouTube Standard Enthalpy Of Formation Gallium Enthalpy of formation (δhf) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements. Standard Enthalpy Of Formation Gallium.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Gallium The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation Gallium.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Gallium Mallard, eds, nist chemistry webbook, nist standard reference database. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation, also known as the heat of formation, is the enthalpy. Standard Enthalpy Of Formation Gallium.
From narodnatribuna.info
Enthalpies Of Formation And Melting Points Of Iiiv Standard Enthalpy Of Formation Gallium The formation of any chemical can be as a reaction from the corresponding elements: The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard, eds, nist chemistry webbook, nist standard reference database. The. Standard Enthalpy Of Formation Gallium.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Gallium The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The formation of any chemical can be as a reaction from the corresponding elements: Standand enthalpies of formation & standard entropies of common compounds substance state ∆h. Standard Enthalpy Of Formation Gallium.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Gallium Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+ aq 105.79. The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. Mallard, eds, nist chemistry webbook, nist standard reference database. Enthalpy of formation (δhf). Standard Enthalpy Of Formation Gallium.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Gallium The standard enthalpy of formation, δh ∘ f, is the enthalpy change accompanying the formation of 1 mole of a substance from the. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms. Standard Enthalpy Of Formation Gallium.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Gallium The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. All standard. Standard Enthalpy Of Formation Gallium.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Gallium All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard conditions. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure. Standard Enthalpy Of Formation Gallium.