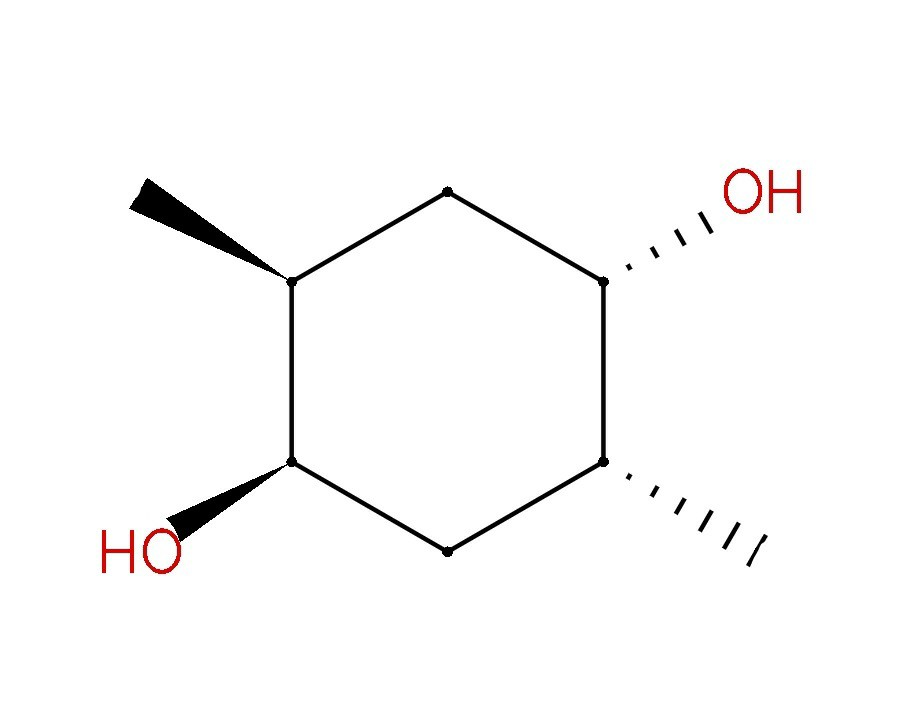
Meso Compounds Organic Chemistry For Dummies . Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. Such compounds, which are achiral, yet. A meso compound contains an internal plane of symmetry. Meso compounds are achiral compounds that has multiple chiral centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. It is superimposed on its mirror image and is optically. Meso compounds (video) | stereochemistry | khan academy A meso compound is an achiral compound that has chiral centers. Mesomers are compounds with zero net rotation of plane polarised light. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can.
from chemistry.stackexchange.com
It is superimposed on its mirror image and is optically. Mesomers are compounds with zero net rotation of plane polarised light. Meso compounds are achiral compounds that has multiple chiral centers. A meso compound contains an internal plane of symmetry. Meso compounds (video) | stereochemistry | khan academy Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Such compounds, which are achiral, yet. A meso compound is an achiral compound that has chiral centers. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers.
organic chemistry Identifying a meso compound Chemistry Stack Exchange
Meso Compounds Organic Chemistry For Dummies A meso compound contains an internal plane of symmetry. A meso compound contains an internal plane of symmetry. Meso compounds are achiral compounds that has multiple chiral centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. It is superimposed on its mirror image and is optically. Mesomers are compounds with zero net rotation of plane polarised light. A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. Such compounds, which are achiral, yet. Meso compounds (video) | stereochemistry | khan academy In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. A meso compound is an achiral compound that has chiral centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers.
From www.masterorganicchemistry.com
The Meso Trap Master Organic Chemistry Meso Compounds Organic Chemistry For Dummies A meso compound is an achiral compound that has chiral centers. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. Such compounds, which are achiral, yet. A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. A meso. Meso Compounds Organic Chemistry For Dummies.
From www.organicchemistrytutor.com
Meso Compounds — Organic Chemistry Tutor Meso Compounds Organic Chemistry For Dummies A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. Mesomers are compounds with zero net rotation of plane polarised light. Meso compounds are achiral compounds that has multiple chiral centers. A meso compound is an achiral compound that has chiral centers. In other words, mesomers are. Meso Compounds Organic Chemistry For Dummies.
From chemistry.stackexchange.com
organic chemistry Conformers of a Chemistry Stack Meso Compounds Organic Chemistry For Dummies Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero. Meso Compounds Organic Chemistry For Dummies.
From chemistry.stackexchange.com
organic chemistry Identifying a meso compound Chemistry Stack Exchange Meso Compounds Organic Chemistry For Dummies A meso compound is an achiral compound that has chiral centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Meso compounds (video) | stereochemistry | khan academy Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. It is superimposed. Meso Compounds Organic Chemistry For Dummies.
From www.chemistrysteps.com
Meso Compounds Chemistry Steps Meso Compounds Organic Chemistry For Dummies A meso compound is an achiral compound that has chiral centers. It is superimposed on its mirror image and is optically. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. A meso. Meso Compounds Organic Chemistry For Dummies.
From chemistry.com.pk
Free Download Organic Chemistry I Workbook for Dummies (2nd Edition) By Meso Compounds Organic Chemistry For Dummies A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. A meso compound contains an internal plane of symmetry. In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. Meso compounds (video) | stereochemistry | khan academy. Meso Compounds Organic Chemistry For Dummies.
From www.youtube.com
Meso Compounds YouTube Meso Compounds Organic Chemistry For Dummies Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Meso compounds (video) | stereochemistry | khan academy A meso compound contains an internal plane of symmetry. A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. A. Meso Compounds Organic Chemistry For Dummies.
From www.dummies.com
Organic Chemistry I For Dummies Cheat Sheet Meso Compounds Organic Chemistry For Dummies Mesomers are compounds with zero net rotation of plane polarised light. Meso compounds (video) | stereochemistry | khan academy Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. A meso compound contains an internal plane of symmetry. Because of the plane of symmetry, the molecule is achiral, despite the fact. Meso Compounds Organic Chemistry For Dummies.
From www.clutchprep.com
Meso Compound Organic Chemistry Video Clutch Prep Meso Compounds Organic Chemistry For Dummies A meso compound contains an internal plane of symmetry. Such compounds, which are achiral, yet. In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. A meso compound is a stereoisomer with. Meso Compounds Organic Chemistry For Dummies.
From todayworldinfo.com
Meso Compound The Different Forms of a Meso Compound Meso Compounds Organic Chemistry For Dummies Meso compounds are achiral compounds that has multiple chiral centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. A meso compound is an achiral compound that has chiral centers. In other. Meso Compounds Organic Chemistry For Dummies.
From sanet.st
Organic Chemistry II For Dummies, 2nd Edition SoftArchive Meso Compounds Organic Chemistry For Dummies A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. Mesomers are compounds with zero net rotation of plane polarised light. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. It is superimposed on its mirror image. Meso Compounds Organic Chemistry For Dummies.
From courses.lumenlearning.com
Meso Compounds MCC Organic Chemistry Meso Compounds Organic Chemistry For Dummies In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. A meso compound is an achiral compound that has chiral centers. Mesomers are compounds with zero net rotation of plane polarised light. Such compounds, which are achiral, yet. A meso compound is a stereoisomer with two or more chiral centres but no. Meso Compounds Organic Chemistry For Dummies.
From www.chemistrysteps.com
Meso Compounds Chemistry Steps Meso Compounds Organic Chemistry For Dummies In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. Meso compounds are achiral compounds that has multiple chiral centers. A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. A meso compound contains an internal plane. Meso Compounds Organic Chemistry For Dummies.
From www.organicchemistrytutor.com
How to Identify Chiral Atoms, Chiral Molecules, and Meso Compounds Meso Compounds Organic Chemistry For Dummies A meso compound is an achiral compound that has chiral centers. A meso compound contains an internal plane of symmetry. Mesomers are compounds with zero net rotation of plane polarised light. Such compounds, which are achiral, yet. Meso compounds are achiral compounds that has multiple chiral centers. Meso compounds (video) | stereochemistry | khan academy In other words, mesomers are. Meso Compounds Organic Chemistry For Dummies.
From fotokolekcija.blogspot.com
Identical Compounds Organic Chemistry Foto Kolekcija Meso Compounds Organic Chemistry For Dummies Mesomers are compounds with zero net rotation of plane polarised light. Such compounds, which are achiral, yet. In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. A meso compound is an. Meso Compounds Organic Chemistry For Dummies.
From www.masterorganicchemistry.com
The Meso Trap Master Organic Chemistry Meso Compounds Organic Chemistry For Dummies Meso compounds are achiral compounds that has multiple chiral centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Mesomers are compounds with zero net rotation of plane polarised light. A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal. Meso Compounds Organic Chemistry For Dummies.
From chemistnotes.com
Meso compounds; Definition and Examples Chemistry Notes Meso Compounds Organic Chemistry For Dummies A meso compound is an achiral compound that has chiral centers. Such compounds, which are achiral, yet. Meso compounds (video) | stereochemistry | khan academy Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In organic chemistry, you need to be able to spot planes of symmetry in molecules so. Meso Compounds Organic Chemistry For Dummies.
From www.masterorganicchemistry.com
The Meso Trap Master Organic Chemistry Meso Compounds Organic Chemistry For Dummies A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. A meso compound is an achiral compound that has chiral centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Meso compounds (video) | stereochemistry | khan. Meso Compounds Organic Chemistry For Dummies.
From www.chegg.com
Solved Which of the following are meso compounds? Meso Compounds Organic Chemistry For Dummies Meso compounds are achiral compounds that has multiple chiral centers. A meso compound is an achiral compound that has chiral centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. It is superimposed on its mirror image and is optically. In organic chemistry, you need to be able to spot. Meso Compounds Organic Chemistry For Dummies.
From www.pinterest.com
MESOFORM Chemistry lessons, Organic chemistry, Organic chem Meso Compounds Organic Chemistry For Dummies A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. It is superimposed on its mirror image and is optically. Meso compounds are achiral compounds that has multiple chiral centers. A meso compound is an achiral compound that has chiral centers. A meso compound contains an internal. Meso Compounds Organic Chemistry For Dummies.
From www.artofit.org
Organic chemistry educational infographics meso compounds and achiral Meso Compounds Organic Chemistry For Dummies Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Meso compounds (video) | stereochemistry | khan academy A meso compound is an achiral compound that has chiral centers. Such compounds, which are achiral, yet. A meso compound is a stereoisomer with two or more chiral centres but no optical activity. Meso Compounds Organic Chemistry For Dummies.
From www.studypool.com
SOLUTION Organic chemistry ii for dummies pdfdrive Studypool Meso Compounds Organic Chemistry For Dummies Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. A meso compound contains an internal plane of symmetry. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. It is superimposed on its mirror image and is optically. A meso compound. Meso Compounds Organic Chemistry For Dummies.
From shimizu-uofsc.net
Identify Meso Molecules Organic Chemistry How to…. Meso Compounds Organic Chemistry For Dummies Such compounds, which are achiral, yet. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In organic chemistry, you need to be able to spot planes of symmetry in molecules so. Meso Compounds Organic Chemistry For Dummies.
From ar.inspiredpencil.com
Meso Compounds Meso Compounds Organic Chemistry For Dummies Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. Meso compounds (video) | stereochemistry | khan academy Because of the plane of symmetry, the molecule is achiral, despite the fact that it. Meso Compounds Organic Chemistry For Dummies.
From www.clutchprep.com
Meso Compound Organic Chemistry Video Clutch Prep Meso Compounds Organic Chemistry For Dummies It is superimposed on its mirror image and is optically. Meso compounds (video) | stereochemistry | khan academy Mesomers are compounds with zero net rotation of plane polarised light. A meso compound contains an internal plane of symmetry. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. Meso compounds are. Meso Compounds Organic Chemistry For Dummies.
From www.studocu.com
Organic Compounds Organic Compounds Organic Chemistry is one of the Meso Compounds Organic Chemistry For Dummies A meso compound is an achiral compound that has chiral centers. Meso compounds are achiral compounds that has multiple chiral centers. A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. A meso compound contains an internal plane of symmetry. Because of the plane of symmetry, the. Meso Compounds Organic Chemistry For Dummies.
From www.chegg.com
Solved 7) How are meso compounds different from nonmeso Meso Compounds Organic Chemistry For Dummies Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. A meso compound is an achiral compound that has chiral centers. A meso compound contains an internal plane of symmetry. Because. Meso Compounds Organic Chemistry For Dummies.
From www.masterorganicchemistry.com
The Meso Trap Master Organic Chemistry Meso Compounds Organic Chemistry For Dummies A meso compound is an achiral compound that has chiral centers. A meso compound contains an internal plane of symmetry. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. Mesomers are compounds with zero net rotation of plane polarised light. In other words, mesomers are organic molecules that have two chiral. Meso Compounds Organic Chemistry For Dummies.
From ar.inspiredpencil.com
Meso Compounds Meso Compounds Organic Chemistry For Dummies It is superimposed on its mirror image and is optically. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In other words, mesomers are organic molecules that have two chiral carbons that. Meso Compounds Organic Chemistry For Dummies.
From chemistry.com.pk
Free Download Chemistry for Dummies All in One By Christopher R. Hren Meso Compounds Organic Chemistry For Dummies A meso compound is an achiral compound that has chiral centers. A meso compound contains an internal plane of symmetry. Such compounds, which are achiral, yet. Meso compounds (video) | stereochemistry | khan academy In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. Because of the plane of symmetry, the molecule. Meso Compounds Organic Chemistry For Dummies.
From www.chemistrysteps.com
Meso Compounds Chemistry Steps Meso Compounds Organic Chemistry For Dummies In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. It is superimposed on its mirror image and is optically. A meso compound is an achiral compound that has chiral centers. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers.. Meso Compounds Organic Chemistry For Dummies.
From www.coursehero.com
[Solved] And if any are meso compounds. Determine the relationship Meso Compounds Organic Chemistry For Dummies Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. It is superimposed on its mirror image and is optically. A meso compound contains an internal plane of symmetry. Such compounds, which are. Meso Compounds Organic Chemistry For Dummies.
From www.organicchemistrytutor.com
How to Identify Chiral Atoms, Chiral Molecules, and Meso Compounds Meso Compounds Organic Chemistry For Dummies Mesomers are compounds with zero net rotation of plane polarised light. A meso compound is a stereoisomer with two or more chiral centres but no optical activity due to an internal plane of symmetry. Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. In organic chemistry, you need to be. Meso Compounds Organic Chemistry For Dummies.
From www.pinterest.co.kr
If you are asked to draw the meso isomer Teaching chemistry Meso Compounds Organic Chemistry For Dummies Because of the plane of symmetry, the molecule is achiral, despite the fact that it has two chirality centers. It is superimposed on its mirror image and is optically. Meso compounds (video) | stereochemistry | khan academy Such compounds, which are achiral, yet. Meso compounds are achiral compounds that has multiple chiral centers. Because of the plane of symmetry, the. Meso Compounds Organic Chemistry For Dummies.
From ar.inspiredpencil.com
Meso Compounds Meso Compounds Organic Chemistry For Dummies In other words, mesomers are organic molecules that have two chiral carbons that are identical, resulting in zero net rotation. In organic chemistry, you need to be able to spot planes of symmetry in molecules so you can. It is superimposed on its mirror image and is optically. Mesomers are compounds with zero net rotation of plane polarised light. Meso. Meso Compounds Organic Chemistry For Dummies.