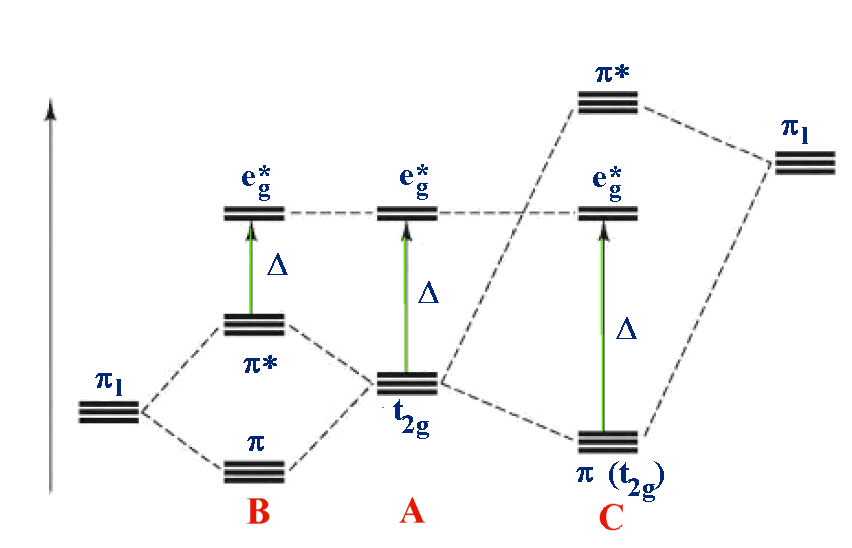
Ligand Field Strength . This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. So the strategy here is to first determine state symmetries followed by applications of ligand field. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. It explains the covalent bond. It allows us to explain the differences between strong.
from chem.libretexts.org
Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. It explains the covalent bond. So the strategy here is to first determine state symmetries followed by applications of ligand field. It allows us to explain the differences between strong. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of.
Ligand Field Theory Fundamentals Chemistry LibreTexts
Ligand Field Strength It explains the covalent bond. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. So the strategy here is to first determine state symmetries followed by applications of ligand field. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. It explains the covalent bond. It allows us to explain the differences between strong.
From www.youtube.com
Trick to learn weak and strong field ligand (spectrochemical series Ligand Field Strength This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. It allows us to explain the differences between strong. Ligands that bind through very electronegative atoms such as o and halogens are thus. Ligand Field Strength.
From chemizi.blogspot.com
Ligandsdefinitionexamplestypes in coordination chemistry Ligand Field Strength This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. So the strategy here is to first determine state symmetries followed by applications of ligand field. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. There are a few factors that determine. Ligand Field Strength.
From www.researchgate.net
Schematic diagram of the ligand field splitting of the highspin and Ligand Field Strength So the strategy here is to first determine state symmetries followed by applications of ligand field. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the. Ligand Field Strength.
From chem.libretexts.org
9.5 Ligand Field Theory Chemistry LibreTexts Ligand Field Strength This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. So the strategy here is to first determine state symmetries followed by applications of ligand field. Ligand field theory is. Ligand Field Strength.
From askfilo.com
Arrangement of ligands in order of increasing field strength. Ligand Field Strength It allows us to explain the differences between strong. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. So the strategy here. Ligand Field Strength.
From www.slideserve.com
PPT Coordination Chemistry II PowerPoint Presentation ID270270 Ligand Field Strength It allows us to explain the differences between strong. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. This chapter covers the. Ligand Field Strength.
From study.com
Spectrochemical Series Definition, Splitting & Ligands Lesson Ligand Field Strength So the strategy here is to first determine state symmetries followed by applications of ligand field. It explains the covalent bond. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. Ligand field theory is an extension of crystal field theory which includes orbital overlap. Ligand Field Strength.
From www.researchgate.net
DFAs by ligand field strength a, Stacked normalized Ligand Field Strength This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. It explains the covalent bond. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron. Ligand Field Strength.
From www.slideserve.com
PPT Alfred Werner 1893 VBT Crystal Field Theory (CFT) Modified CFT Ligand Field Strength There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. It allows us to explain the differences between strong. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. This chapter covers the. Ligand Field Strength.
From www.youtube.com
Strong and Weak Field Ligands Coordination Chemistry YouTube Ligand Field Strength So the strategy here is to first determine state symmetries followed by applications of ligand field. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. It allows us to explain the differences between strong. There are a few factors that determine the magnitude of the d orbital. Ligand Field Strength.
From cbsenotes.weebly.com
Coordination Compounds NCERT & CBSE Resources Ligand Field Strength So the strategy here is to first determine state symmetries followed by applications of ligand field. It allows us to explain the differences between strong. It explains the covalent bond. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. Ligands that bind through very electronegative atoms such as o and halogens are thus expected. Ligand Field Strength.
From www.slideserve.com
PPT Applications of UV/VIS PowerPoint Presentation, free download Ligand Field Strength It allows us to explain the differences between strong. So the strategy here is to first determine state symmetries followed by applications of ligand field. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy. Ligand Field Strength.
From chem.libretexts.org
Ligand Field Theory Fundamentals Chemistry LibreTexts Ligand Field Strength There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. So the strategy here is to first determine state symmetries followed by applications. Ligand Field Strength.
From www.scribd.com
Ligand Field Strength PDF Ligand Coordination Complex Ligand Field Strength It allows us to explain the differences between strong. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. So the strategy here is to first determine state symmetries followed by applications of ligand field. There are a few factors that determine the magnitude of the d orbital. Ligand Field Strength.
From studylib.net
Ligand field theory Ligand Field Strength It explains the covalent bond. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. So the strategy here is to first determine. Ligand Field Strength.
From www.youtube.com
CHEM102 CH19 pt15 ligand field strength YouTube Ligand Field Strength It allows us to explain the differences between strong. It explains the covalent bond. So the strategy here is to first determine state symmetries followed by applications of ligand field. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. Ligands that bind through very electronegative atoms such. Ligand Field Strength.
From pubs.rsc.org
Tuning ligand field strength with pendent Lewis acids access to high Ligand Field Strength Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. It explains the covalent bond. So the strategy here is to first determine. Ligand Field Strength.
From revistas.unam.mx
volumen 33 número 1 Ligand Field Strength It allows us to explain the differences between strong. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. It explains the covalent bond. So the strategy here is to first determine state symmetries followed by applications of ligand field. There are a few factors that determine the magnitude of the d orbital splitting, and. Ligand Field Strength.
From www.slideserve.com
PPT Chapter 24 Transition Metals and Coordination Compounds Ligand Field Strength This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. So the strategy here is to first determine state symmetries followed by applications of ligand field. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. Ligand field theory is. Ligand Field Strength.
From www.slideserve.com
PPT CHEM 522 Chapter 01 PowerPoint Presentation, free download ID Ligand Field Strength Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. So the strategy here is to first determine state symmetries followed by applications of ligand field. It explains the covalent bond. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. Ligands that. Ligand Field Strength.
From www.youtube.com
Ligand Field Theory Part 1 LFT Molecular Orbital Theory ACFT Ligand Field Strength Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. It explains the covalent bond. So the strategy here is to first determine. Ligand Field Strength.
From www.slideserve.com
PPT CHEM 522 Chapter 01 PowerPoint Presentation, free download ID Ligand Field Strength It explains the covalent bond. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. So the strategy here is to first determine state symmetries followed by applications of ligand field. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set. Ligand Field Strength.
From chem.libretexts.org
Ligand Field Theory Fundamentals Chemistry LibreTexts Ligand Field Strength This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. It explains the covalent bond. Ligand field theory is an extension of crystal field theory which includes orbital overlap between. Ligand Field Strength.
From chem.libretexts.org
9.5 Ligand Field Theory Chemistry LibreTexts Ligand Field Strength It explains the covalent bond. So the strategy here is to first determine state symmetries followed by applications of ligand field. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through. Ligand Field Strength.
From www.slideserve.com
PPT Coordination Chemistry II PowerPoint Presentation ID270270 Ligand Field Strength This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind. Ligand Field Strength.
From www.slideserve.com
PPT Electronic Structure of dMetal Complexes Ligand Field Theory Ligand Field Strength Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. It explains the covalent bond. So the strategy here is to first determine. Ligand Field Strength.
From www.slideserve.com
PPT Coordination Chemistry II PowerPoint Presentation, free download Ligand Field Strength Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. So the strategy here is to first determine state symmetries followed by applications. Ligand Field Strength.
From www.researchgate.net
DFAs by ligand field strength. a, Stacked normalized Ligand Field Strength Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. It allows us to explain the differences between strong. There are a few factors that determine the magnitude of the d orbital splitting,. Ligand Field Strength.
From pubs.acs.org
Density Functional Theory (DFT)Based Bonding Analysis Correlates Ligand Field Strength Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. It explains the covalent bond. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. So the strategy here is to first determine state symmetries followed by applications of ligand field. There are. Ligand Field Strength.
From www.chegg.com
Solved Arrange the ligands in order of increasing ligand Ligand Field Strength So the strategy here is to first determine state symmetries followed by applications of ligand field. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field,. Ligand Field Strength.
From www.researchgate.net
Increasing ligand field strength by alleviating steric strain in Ligand Field Strength Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. It explains the covalent bond. It allows us to explain the differences between strong. Ligands that bind through very electronegative atoms such as. Ligand Field Strength.
From chem.libretexts.org
Introduction to Ligand Field Theory Chemistry LibreTexts Ligand Field Strength So the strategy here is to first determine state symmetries followed by applications of ligand field. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. It allows us to explain the differences between strong. It explains the covalent bond. Ligand field theory is an. Ligand Field Strength.
From www.researchgate.net
Figure S2. Ligand field strength per ligand for each model complex Ligand Field Strength It explains the covalent bond. There are a few factors that determine the magnitude of the d orbital splitting, and whether an electron can occupy the higher energy set of. It allows us to explain the differences between strong. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d. Ligand Field Strength.
From www.slideserve.com
PPT Alfred Werner 1893 VBT Crystal Field Theory (CFT) Modified CFT Ligand Field Strength It allows us to explain the differences between strong. Ligands that bind through very electronegative atoms such as o and halogens are thus expected to be weak field, and ligands that bind through c. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. This chapter covers the. Ligand Field Strength.
From www.slideserve.com
PPT 251 Werner’s Theory of Coordination Compounds An Overview Ligand Field Strength This chapter covers the basic concepts of ligand structure, bonding, and nomenclature in metal chemistry. It allows us to explain the differences between strong. It explains the covalent bond. Ligand field theory is an extension of crystal field theory which includes orbital overlap between ligand orbitals and the metal d orbitals. Ligands that bind through very electronegative atoms such as. Ligand Field Strength.