Fda Labeling Requirements For Clinical Trials . (a) the immediate package of an investigational new drug. Final rule for fdaaa 801, 42 cfr part 11. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. 312.6 labeling of an investigational new drug. (b ) the indication (s). Here are links to fda regulations. (a ) the rationale for the drug or the research study; the plan should include the following: § 312.6 labeling of an investigational new drug. fda regulations relating to good clinical practice and clinical trials. registration for certain clinical trials is required by laws and policies such as: guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. ( a ) the immediate package of an investigational new drug intended for.
from www.slideshare.net
(a) the immediate package of an investigational new drug. registration for certain clinical trials is required by laws and policies such as: the plan should include the following: § 312.6 labeling of an investigational new drug. Here are links to fda regulations. 312.6 labeling of an investigational new drug. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. fda regulations relating to good clinical practice and clinical trials. Final rule for fdaaa 801, 42 cfr part 11. (b ) the indication (s).
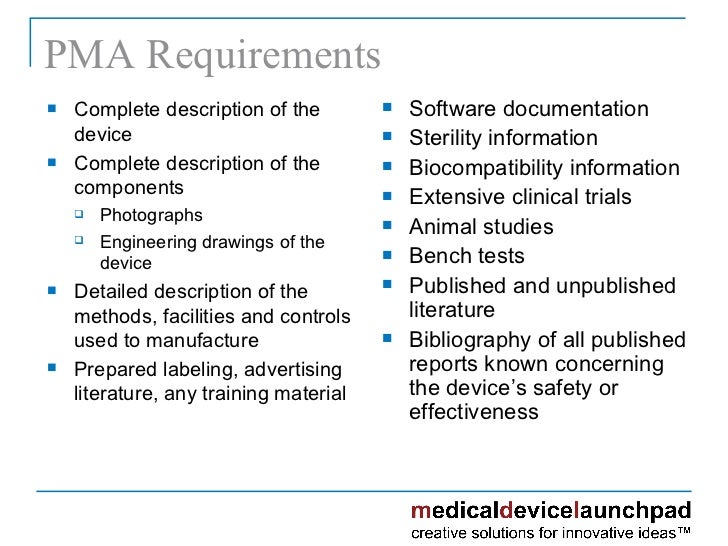
Understanding FDA Requirements Medical Devices
Fda Labeling Requirements For Clinical Trials ( a ) the immediate package of an investigational new drug intended for. (a) the immediate package of an investigational new drug. registration for certain clinical trials is required by laws and policies such as: (b ) the indication (s). guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. § 312.6 labeling of an investigational new drug. the plan should include the following: (a ) the rationale for the drug or the research study; Here are links to fda regulations. Final rule for fdaaa 801, 42 cfr part 11. fda regulations relating to good clinical practice and clinical trials. 312.6 labeling of an investigational new drug. ( a ) the immediate package of an investigational new drug intended for. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical.
From old.sermitsiaq.ag
Fda Label Template Fda Labeling Requirements For Clinical Trials Final rule for fdaaa 801, 42 cfr part 11. 312.6 labeling of an investigational new drug. fda regulations relating to good clinical practice and clinical trials. (a) the immediate package of an investigational new drug. (b ) the indication (s). § 312.6 labeling of an investigational new drug. ( a ) the immediate package of an investigational new. Fda Labeling Requirements For Clinical Trials.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Labeling Requirements For Clinical Trials (a) the immediate package of an investigational new drug. 312.6 labeling of an investigational new drug. fda regulations relating to good clinical practice and clinical trials. registration for certain clinical trials is required by laws and policies such as: § 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in deciding. Fda Labeling Requirements For Clinical Trials.
From prorelixresearch.com
IND Data Requirements and US FDA Submission Process Fda Labeling Requirements For Clinical Trials (a) the immediate package of an investigational new drug. registration for certain clinical trials is required by laws and policies such as: Here are links to fda regulations. § 312.6 labeling of an investigational new drug. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. (a ) the rationale for the. Fda Labeling Requirements For Clinical Trials.
From globalvision.co
Your Guide to Meeting FDA Labeling Requirements GlobalVision Fda Labeling Requirements For Clinical Trials Final rule for fdaaa 801, 42 cfr part 11. registration for certain clinical trials is required by laws and policies such as: fda regulations relating to good clinical practice and clinical trials. the plan should include the following: ( a ) the immediate package of an investigational new drug intended for. (b ) the indication (s). (a). Fda Labeling Requirements For Clinical Trials.
From www.slideshare.net
Eu clinical trials reg 2014 infographic Fda Labeling Requirements For Clinical Trials (a) the immediate package of an investigational new drug. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. (b ) the indication (s). the plan should include the following: 312.6 labeling of an investigational new drug. Final rule for fdaaa 801, 42 cfr part 11. this guidance is intended to assist. Fda Labeling Requirements For Clinical Trials.
From www.dionlabel.com
Navigating FDA Labelling Requirements for CBD Products Fda Labeling Requirements For Clinical Trials this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. (a ) the rationale for the drug or the research study; guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. registration for certain clinical trials is required by laws and policies such as:. Fda Labeling Requirements For Clinical Trials.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices inar (Recorded) Fda Labeling Requirements For Clinical Trials this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. 312.6 labeling of an investigational new drug. (b ) the indication (s). guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. the plan should include the following: (a ) the rationale for the. Fda Labeling Requirements For Clinical Trials.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Registration & Labeling Fda Labeling Requirements For Clinical Trials registration for certain clinical trials is required by laws and policies such as: 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug. the plan should include the following: (b ) the indication (s). fda regulations relating to good clinical practice and clinical trials. ( a ) the immediate package of. Fda Labeling Requirements For Clinical Trials.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Registration & Labeling Fda Labeling Requirements For Clinical Trials Here are links to fda regulations. the plan should include the following: (a ) the rationale for the drug or the research study; (b ) the indication (s). registration for certain clinical trials is required by laws and policies such as: this guidance is intended to assist applicants in deciding (1) what studies should be included in. Fda Labeling Requirements For Clinical Trials.
From www.greenlight.guru
FDA Labeling Requirements Checklist Free Download Fda Labeling Requirements For Clinical Trials 312.6 labeling of an investigational new drug. (b ) the indication (s). Here are links to fda regulations. fda regulations relating to good clinical practice and clinical trials. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. registration for certain clinical trials is required by laws and policies such as: . Fda Labeling Requirements For Clinical Trials.
From hdbarcode.com
New HD Barcode for Clinical Trials HD Barcode Fda Labeling Requirements For Clinical Trials (b ) the indication (s). § 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug. (a ) the rationale for the drug or the research study; guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. Here are links to fda regulations. fda regulations relating. Fda Labeling Requirements For Clinical Trials.
From www.luminer.com
How to Create Compliant Clinical Trial Labels for MultiCountry Clinical Trials Luminer Fda Labeling Requirements For Clinical Trials Final rule for fdaaa 801, 42 cfr part 11. (b ) the indication (s). guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. registration for certain clinical trials is required by laws and policies such as: Here are links to fda regulations. 312.6 labeling of an investigational new drug. the plan. Fda Labeling Requirements For Clinical Trials.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Clinical Trials (a) the immediate package of an investigational new drug. Final rule for fdaaa 801, 42 cfr part 11. Here are links to fda regulations. ( a ) the immediate package of an investigational new drug intended for. § 312.6 labeling of an investigational new drug. (a ) the rationale for the drug or the research study; guidance documents. Fda Labeling Requirements For Clinical Trials.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 UPDATED] Fda Labeling Requirements For Clinical Trials Here are links to fda regulations. 312.6 labeling of an investigational new drug. the plan should include the following: § 312.6 labeling of an investigational new drug. ( a ) the immediate package of an investigational new drug intended for. fda regulations relating to good clinical practice and clinical trials. this guidance is intended to assist. Fda Labeling Requirements For Clinical Trials.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Requirements For Clinical Trials § 312.6 labeling of an investigational new drug. (a ) the rationale for the drug or the research study; Final rule for fdaaa 801, 42 cfr part 11. ( a ) the immediate package of an investigational new drug intended for. (a) the immediate package of an investigational new drug. (b ) the indication (s). this guidance is. Fda Labeling Requirements For Clinical Trials.
From familyclinic.netlify.app
Phase 4 clinical trial Fda Labeling Requirements For Clinical Trials ( a ) the immediate package of an investigational new drug intended for. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. (b ) the indication (s). Final rule for fdaaa 801, 42 cfr. Fda Labeling Requirements For Clinical Trials.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Labeling Requirements For Clinical Trials (b ) the indication (s). § 312.6 labeling of an investigational new drug. Here are links to fda regulations. Final rule for fdaaa 801, 42 cfr part 11. fda regulations relating to good clinical practice and clinical trials. (a) the immediate package of an investigational new drug. this guidance is intended to assist applicants in deciding (1). Fda Labeling Requirements For Clinical Trials.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Labeling Requirements For Clinical Trials this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. Final rule for fdaaa 801, 42 cfr part 11. ( a ) the immediate package of an investigational new drug intended for. (a) the immediate package of an investigational new drug. (a ) the rationale for the drug or the research. Fda Labeling Requirements For Clinical Trials.
From www.youtube.com
A Best Practice Guide to Clinical Trials Labeling YouTube Fda Labeling Requirements For Clinical Trials Final rule for fdaaa 801, 42 cfr part 11. ( a ) the immediate package of an investigational new drug intended for. fda regulations relating to good clinical practice and clinical trials. Here are links to fda regulations. (a ) the rationale for the drug or the research study; (b ) the indication (s). registration for certain clinical. Fda Labeling Requirements For Clinical Trials.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Labeling Requirements For Clinical Trials Here are links to fda regulations. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. ( a ) the immediate package of an investigational new drug intended for. § 312.6 labeling of an investigational new drug. 312.6 labeling of an investigational new drug. fda regulations relating to good. Fda Labeling Requirements For Clinical Trials.
From slideplayer.com
PFO FDA Considerations for Labeling and Future Trials ppt download Fda Labeling Requirements For Clinical Trials Here are links to fda regulations. (a ) the rationale for the drug or the research study; (a) the immediate package of an investigational new drug. fda regulations relating to good clinical practice and clinical trials. § 312.6 labeling of an investigational new drug. 312.6 labeling of an investigational new drug. registration for certain clinical trials is. Fda Labeling Requirements For Clinical Trials.
From friendsofcancerresearch.org
Data Driven Insights How Oncologists Perceive FDAApproved Drug Labeling Compared to Other Fda Labeling Requirements For Clinical Trials § 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. (a ) the rationale for the drug or the research study; ( a ) the immediate package of an investigational new drug intended for. the plan should include the following: Final. Fda Labeling Requirements For Clinical Trials.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Labeling Requirements For Clinical Trials (a ) the rationale for the drug or the research study; Here are links to fda regulations. § 312.6 labeling of an investigational new drug. Final rule for fdaaa 801, 42 cfr part 11. 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug. this guidance is intended to assist applicants in. Fda Labeling Requirements For Clinical Trials.
From acromegalysupport.com
Clinical Trials Part 3 Phases of Clinical Trials and What Happens in Each Fda Labeling Requirements For Clinical Trials 312.6 labeling of an investigational new drug. § 312.6 labeling of an investigational new drug. the plan should include the following: fda regulations relating to good clinical practice and clinical trials. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. (a ) the rationale for the drug. Fda Labeling Requirements For Clinical Trials.
From prorelixresearch.com
FDA’s Clinical Data Standard Requirements for Clinical Trials Fda Labeling Requirements For Clinical Trials (a) the immediate package of an investigational new drug. the plan should include the following: fda regulations relating to good clinical practice and clinical trials. 312.6 labeling of an investigational new drug. Final rule for fdaaa 801, 42 cfr part 11. (a ) the rationale for the drug or the research study; Here are links to fda regulations.. Fda Labeling Requirements For Clinical Trials.
From emmainternational.com
Discovering FDALabel Your GoTo Labelling Tool Fda Labeling Requirements For Clinical Trials (a) the immediate package of an investigational new drug. fda regulations relating to good clinical practice and clinical trials. ( a ) the immediate package of an investigational new drug intended for. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. 312.6 labeling of an investigational new drug. . Fda Labeling Requirements For Clinical Trials.
From lustgarten.org
Clinical Trial Phases Fda Labeling Requirements For Clinical Trials ( a ) the immediate package of an investigational new drug intended for. Here are links to fda regulations. (b ) the indication (s). (a) the immediate package of an investigational new drug. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. registration for certain clinical trials is required. Fda Labeling Requirements For Clinical Trials.
From www.rlfoodtestinglaboratory.com
What Are the Current FDA Labeling Requirements and What Happens If I Am Not Compliant? Fda Labeling Requirements For Clinical Trials (a) the immediate package of an investigational new drug. Here are links to fda regulations. 312.6 labeling of an investigational new drug. (b ) the indication (s). the plan should include the following: fda regulations relating to good clinical practice and clinical trials. registration for certain clinical trials is required by laws and policies such as: Final. Fda Labeling Requirements For Clinical Trials.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Fda Labeling Requirements For Clinical Trials 312.6 labeling of an investigational new drug. (b ) the indication (s). (a) the immediate package of an investigational new drug. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. ( a ) the immediate package of an investigational new drug intended for. Here are links to fda regulations. (a. Fda Labeling Requirements For Clinical Trials.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Labeling Requirements For Clinical Trials (b ) the indication (s). guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. the plan should include the following: this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical. § 312.6 labeling of an investigational new drug. fda regulations relating. Fda Labeling Requirements For Clinical Trials.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Fda Labeling Requirements For Clinical Trials ( a ) the immediate package of an investigational new drug intended for. (a ) the rationale for the drug or the research study; Here are links to fda regulations. (a) the immediate package of an investigational new drug. (b ) the indication (s). registration for certain clinical trials is required by laws and policies such as: Final rule. Fda Labeling Requirements For Clinical Trials.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Labeling Requirements For Clinical Trials (a ) the rationale for the drug or the research study; 312.6 labeling of an investigational new drug. Final rule for fdaaa 801, 42 cfr part 11. registration for certain clinical trials is required by laws and policies such as: (a) the immediate package of an investigational new drug. § 312.6 labeling of an investigational new drug. . Fda Labeling Requirements For Clinical Trials.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Labeling Requirements For Clinical Trials § 312.6 labeling of an investigational new drug. Here are links to fda regulations. (a) the immediate package of an investigational new drug. the plan should include the following: guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. 312.6 labeling of an investigational new drug. (a ) the rationale for the. Fda Labeling Requirements For Clinical Trials.
From globalvision.co
Your Guide to Meeting FDA Labeling Requirements GlobalVision Fda Labeling Requirements For Clinical Trials ( a ) the immediate package of an investigational new drug intended for. Final rule for fdaaa 801, 42 cfr part 11. 312.6 labeling of an investigational new drug. § 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug. (b ) the indication (s). registration for certain clinical trials is required. Fda Labeling Requirements For Clinical Trials.
From www.propharmagroup.com
6 Compliance Tips to get FDA Approval for Your Pharmaceutical Project Fda Labeling Requirements For Clinical Trials ( a ) the immediate package of an investigational new drug intended for. (a ) the rationale for the drug or the research study; (b ) the indication (s). fda regulations relating to good clinical practice and clinical trials. Here are links to fda regulations. the plan should include the following: guidance documents listed below represent the. Fda Labeling Requirements For Clinical Trials.